Detecting a new assembly state of α-synuclein using mass photometry
Work done in the lab of Prof. Alexander Kai Buell, at the Technical University of Denmark (DTU).
Soumik Ray is a Marie Curie postdoctoral fellow at the Bioengineering department of Technical University of Denmark. He did his Master’s degree in Biochemistry from University of Hyderabad (2015). He finished his Ph.D. in 2021 from IIT-Bombay. He is currently investigating the cause and effects of phase separation by intrinsically disordered proteins with a special focus on α-synuclein, the protein involved in the pathogenesis of Parkinson’s disease.
Author Interview
How would you explain your research outcomes to the non-scientific community?
Background:
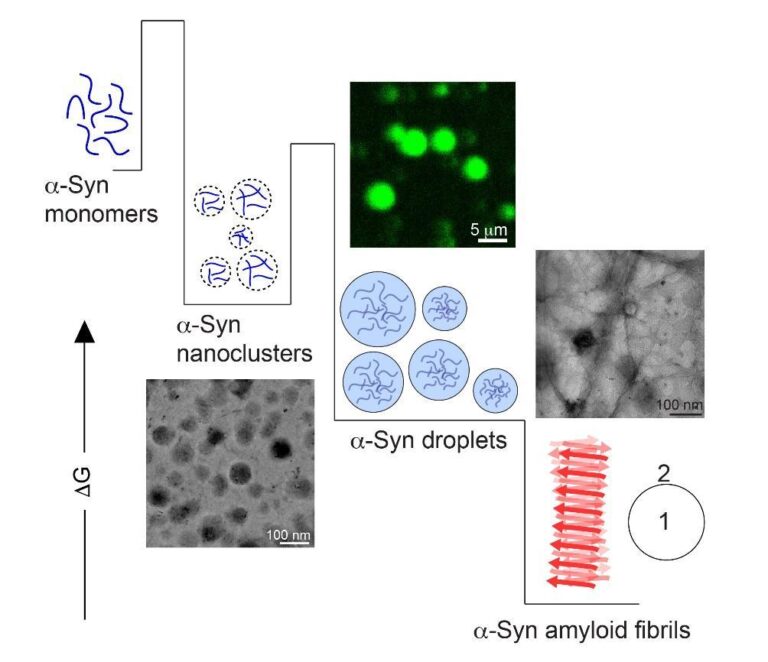
α-Synuclein is a disordered, neuronal protein, which can aggregate into ordered, fibrillar structures called amyloids. Formation of amyloid fibrils is believed to be the major hallmark of Parkinson’s disease pathogenesis. Multiple pathways can trigger α-synuclein monomers to assemble into amyloid fibrils. However, α-synuclein monomers usually need a suitable surface/interface on which they can start to aggregate (we call it the nucleation of amyloid aggregation). These can be as simple as the air-water interface of a test tube, to complex lipid membranes and hydrophobic-hydrophilic interfaces.
During my Ph.D. at IIT Bombay under Prof. Samir K. Maji, we discovered a new pathway that can efficiently nucleate α-synuclein amyloid aggregation independent of surfaces/interfaces. This was via formation of phase separated spherical assemblies (like oil droplets in water). Such α-synuclein assemblies (or droplets) are macroscopic entities (few micrometres in size), which can be visualized under state-of-the-art microscopes and/or using static light scattering. Inside these droplets, the concentration of α-synuclein is extremely high (likely tens of millimolar), which eventually results in homogeneous nucleation of amyloid aggregation within these compartments.
Phase separation occurs above a certain concentration of the protein (termed as the saturation concentration, Csat) at a given solution condition (temperature, pH and ionic strength). To our surprise, we noticed that α-synuclein could form macroscopic droplets even below the experimentally obtained Csat, however with a delayed kinetics (hours before they were detected). This was a bit puzzling because the fundamental concepts of protein phase separation define Csat as a boundary condition below which, one does not expect to achieve macroscopic phase separation at all.
Discovery:
During my postdoctoral tenure at the Technical University of Denmark under Prof. Alexander K. Buell, we embarked on the journey to solve this mystery. Alexander envisioned that there could be instantaneous formation of nanoscopic α-synuclein assemblies below Csat, which grows very slowly and are very few in numbers. This explained why they were invisible to traditional microscopic or light scattering approaches. This was the time when the so-called ‘pre-nucleation clusters’ were also discovered for Tau and FUS proteins (two other disordered proteins that phase separate). Coincidentally, Alexander had visited his old colleague, Nikolai Lorenzen at Novo Nordisk where he was introduced to mass photometry (Refeyn). The OneMP version of this instrument allows label-free, single molecule mass measurements in the range of ~40kDa-5MDa. This was exactly what we needed! The cut-off was conveniently placed so that monomeric α-synuclein (~15kDa) molecules were invisible in the concentration regime in which we wanted to probe the existence of pre-nucleation clusters (if any). Meaning that small α-synuclein assemblies larger than 40kDa could be detected and quantified without the background signal from the monomeric population.
In November 2021, we had our first ‘eureka’ moment. Sub-saturated concentrations of α-synuclein where we could not detect macroscopic phase separation for hours/days, showed spontaneous (within seconds) formation of nanoscale clusters in mass photometry. These nanoclusters had a defined existence boundary in the sub-saturated regime and contained tens to hundreds of molecules depending on total α-synuclein concentration. These assemblies responded to external tweaks the same way as a macroscopic droplet would. Importantly, the nanoclusters were thermodynamically less stable than oligomers—making a strong case for them being phase separated assemblies, just smaller.
“We establish mass photometry as a promising new tool to detect and quantify pre-nucleation clusters in sub-saturated concentrations, which is now emerging as a rather generic phenomenon for proteins that undergo phase separation.”
How do these findings contribute to your research area?
As I mentioned before, until recently it was believed that nucleation of α-synuclein amyloid aggregation was exclusively surface (e.g., lipid membranes) or interface (e.g., air-water or oil-water) dependent. This exclusivity was challenged when we discovered that α-synuclein could undergo phase separation, which opened up a new pathway of amyloid fibril formation associated with Parkinson’s disease. However, the concentration at which the protein phase separated in vitro (hundreds of µM) was one order of magnitude more than what is found in neuronal cells (few µM at the presynaptic termini). Now we show that α-synuclein can undergo nanoscale phase separation at physiological concentrations (we could detect them down to 10 µM total protein concentration). Most importantly, these nanoclusters, despite being few in numbers, can efficiently accelerate amyloid fibril formation of the remaining monomers in solution. This particular observation might have crucial pathological significance with respect to not only Parkinson’s disease, but also all other α-synuclein-related disorders. In addition, we establish mass photometry as a promising new tool to detect and quantify pre-nucleation clusters in sub-saturated concentrations, which is now emerging as a rather generic phenomenon for proteins that undergo phase separation.
What was the exciting moment during your research?
The most exciting moment was when we first saw the nanoclusters in mass photometry in November 2021. Alexander and I went to Novo Nordisk to perform a few experiments and test the instrument for some unrelated work. We did not really expect to obtain any direct evidence of α-synuclein nanoclusters and it was more like a shot-in-the-dark, which did hit the target.
What do you hope to do next?
I think the discovery of prenucleation clusters of α-synuclein opens up a completely new area of research. Nanoscopic size and sparse population make them hard to detect. They cannot be isolated from the solution since it will shift the monomer-nanocluster equilibrium—leading to their dissolution. At this point, I feel that we are somewhat limited by technology to quantitatively study such dynamic, unstable assemblies in depth. However, recent advancements in angstrom resolution microscopy, DNA Paint, small angle X-ray scattering and Cryo electron microscopy surely give us hope. In the coming years, my aim is to probe the existence and causalities of pre-nucleation clusters formed by α-synuclein (and other aggregation prone disordered proteins) in live neuronal cells.
Where do you seek scientific inspiration?
It took us only 66 years from the time we learnt to fly to send astronauts on the moon. Remarkable feats such as this are scattered throughout the scientific history of our species. I try to gather inspiration from stories like these. Especially when my experiments keep failing.
How do you intend to help Indian science improve?
I am strongly inclined to stay in academia. For the next step in my career, I am making an effort to move into more therapeutic and application-oriented research that demands substantial collaborative efforts. I certainly believe that we can achieve significant advancements in the field of protein science through active collaborations with my Indian colleagues and friends.
Reference: Ray, S., Mason, T.O., Boyens-Thiele, L. et al. Mass photometric detection and quantification of nanoscale α-synuclein phase separation. Nat. Chem. 15, 1306–1316 (2023). https://doi.org/10.1038/s41557-023-01244-8
Edited by: Anjali Mahilkar
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.

