Cryo-tomography captures the fascinating infection organelle of tiny parasites
Work done in the lab of Dr. Jonas Barandun and Dr. Lars-Anders Carlson at Umeå University, Sweden.
Himanshu Sharma is a postdoctoral fellow at the MIMS and Umeå University, Sweden. He was awarded the prestigious Marie Curie Postdoctoral fellowship by the European Union for studying Eukaryotic parasites. He currently utilizes cryo-electron Tomography and Single particle analysis to study the infection mechanism of Microsporidia and Viruses. Before moving to Sweden, Himanshu did his PhD at IIT Guwahati, where he utilized biochemical, transcriptomic, and genetic methods to study bacterial ribosome biogenesis.
Author Interview
How would you explain your research outcomes to the non-scientific community?
In this work, we study the infection apparatus of Microsporidia parasites using cryogenic-electron Tomography (cryo-ET). These parasites infect humans and most agriculturally important organisms, thus posing emergent challenges for healthcare, the environment, and the economy.
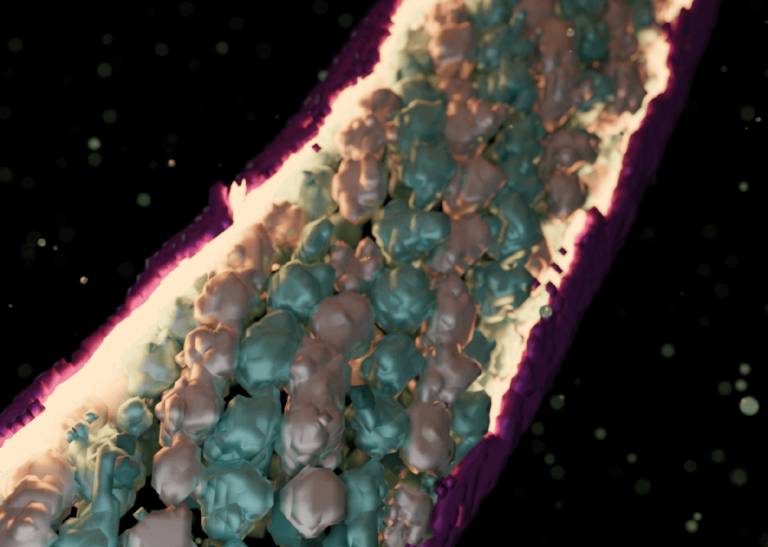
To initiate infection, Microsporidia fires a long tube-like structure called the polar tube (PT), that injects the infective cellular material into the host cell. Understanding this mysteriously fascinating organelle remains elusive as its structure and its firing triggers are not known, and several challenges exist in capturing the infection process in a natural setting. To overcome these problems, we employ cryo-ET to study events leading up to host cell invasion. The methodology enabled us to capture events of host invasion at cryogenic temperatures and generate 3-dimensional volumes of the tube and its transported infectious cargo during the firing events. The method combined with structural analysis using subtomogram averaging revealed diverse interesting states of the polar tube and the transported infective material, thus helping us understand how different components of the polar tube enable host cell invasion.
Our work captures unique states of the infectious cargo including regularly arranged arrays of hibernating ribosomes, the first to be seen across eukaryotes. We also show how the proteins forming the polar tube undergo large-scale changes to enable the tube to transform from a relaxed empty state to a stretched, filled state. Overall, our work provides the first insights into the remodeling of the polar tube components to enable host cell invasion and captures fascinating states of the infectious cargo just before entering the host cells.
“Our work makes important advances in this field by providing first in situ structural insights into the remodeling of the polar tube protein layers when infectious material is injected into the host cells. “
How do these findings contribute to your research area?
The polar tube has been studied for many decades. It is known to be composed of several proteins but no structural information on the proteins and their interactions to form the tube is available to date. This is a huge challenge as the polar tube is the primary mode of infection of these parasites and thus limits any mechanistic studies into how polar tube proteins enable elastic changes in the tube for invading host cells. Our work makes important advances in this field by providing first in situ structural insights into the remodeling of the polar tube protein layers when infectious material is injected into the host cells. Additionally, the unique state of hibernating ribosomes captured inside the tubes presents a unique organization and mode of ribosome regulation parasites and, likely in all eukaryotes.
What was the exciting moment during your research?
This is one of the first instances of utilizing cryo-ET to study microsporidia pathogens. Hence there were several AHA!! moments during our experiments and data analysis stages. One of the most exciting instances occurred when we discovered the regularly arranged pattern of particles in the polar tubes and this immediately set us on a path to identify them using subtomogram averaging. Though performing this analysis was much more difficult than we initially anticipated, but when we discovered that the particles were ribosomes locked in an inactive state, it immediately excited us to understand the biological relevance of these regular packing. Similarly, the changes observed in polar tube proteins also got us extremely captivated in understanding how the tube changes in response to the presence of infectious material during transport.
What do you hope to do next?
For me, this is just the beginning. I aim to follow up on this work by pursuing high-resolution structural studies on individual polar tube proteins. This approach will help us assemble the larger puzzle i.e., the polar tube, and understand the transitions in individual proteins that aid infection, using high-resolution structural studies. In the long term, my objective will be to develop inhibitors of polar tube firing and remodeling to help fight infection in humans, important livestock, and pollinators.
Where do you seek scientific inspiration from?
I enjoy reading very broadly in areas ranging from life science to technology, and other allied subjects. It’s always good to cross-pollinate ideas and get inspiration from the works of others about solving challenges we face in our experimental systems.
How do you intend to help Indian science improve?
Any contribution to a subject area is expected to collectively uplift the science, paving the way for newer and more challenging questions. In the Indian context, microsporidia are severely underreported and rarely studied despite clinical reports of infection in both immunocompetent and immunosuppressed patients. Further, microsporidia infections pose a great threat to agricultural sectors like the silk industry, fishing, and honeybee farming. My next efforts will be to bring in my expertise with these organisms and structural methods to uncover their prevalence and infection mechanisms.
Reference: Sharma H, Jespersen N, Ehrenbolger K, Carlson LA, Barandun J (2024) Ultrastructural insights into the microsporidian infection apparatus reveal the kinetics and morphological transitions of polar tube and cargo during host cell invasion. PLOS Biology 22(2): e3002533. https://doi.org/10.1371/journal.pbio.3002533
The article is also chosen for the cover issue of the current Plos Biology issue. The link is here: https://journals.plos.org/plosbiology/issue?id=10.1371/issue.pbio.v22.i02
Biopatrika: Bringing Science to Society
© Biopatrika 2024 All Rights Reserved.
