FAPP: Filter-Assisted Protein Precipitation sample pre-treatment to enhance bottom-up proteomics
Work done in the lab of Prof. Anurag Singh Rathore at the Centre of Excellence for Biopharmaceutical Technology, Department of Chemical Engineering, Indian Institute of Technology, Delhi (IITD).
Sanghati Bhattacharya, Ph.D., is an institute post-doctoral fellow at the Indian Institute of Technology, Delhi (IITD) and is working on assessment of critical quality attribute and multi-dimensional multi-attribute monitoring of biotherapeutics to improve the characterization and development of numerous biologics. Dr. Bhattacharya has completed her PhD from the University of North Bengal and has several years of professional experience in the characterization of biologics. Dr. Bhattacharya is familiar with a broad range of biophysical methods that are used in the investigation of proteins and other biological therapeutics. Dr. Bhattacharya is a member of the editorial board of an international bioinformatics magazine, has published numerous research articles and reviews, and has won numerous funding to attain national and international conferences.

Author Interview
How would you explain your research outcomes to the non-scientific community?
In the current study, we have developed a sample pre-processing protocol for protein digestion, and the outcome has significant implications for several areas of the biopharmaceutical/ biotherapeutic domains.
Of late, the biopharmaceutical market has been growing in interest with the development of biotherapeutics, in particular monoclonal antibodies (mAbs), which are used to treat, diagnose, or prevent a wide range of illnesses and medical disorders. This has also had a great impact on the foundation of the development of biosimilars by establishing the analytical and functional comparability of highly similar copies of licenced biologic medications to gain the potential cost reduction on healthcare systems. As a result, this could provide more access to biologic medications through the biopharmaceutical industry.
Bottom-up proteomics/protein digestion is one of the most crucial areas of research to achieve this goal because it aids in sequence confirmation, post-translational modification identification, contaminant detection, batch-to-batch consistency assurance, mapping epitopes, PK/PD research, and comparative analyses. Which all work together to advance the development, characterization, and quality control of biotherapeutics, ultimately improving their safety, efficacy, and therapeutic effectiveness.
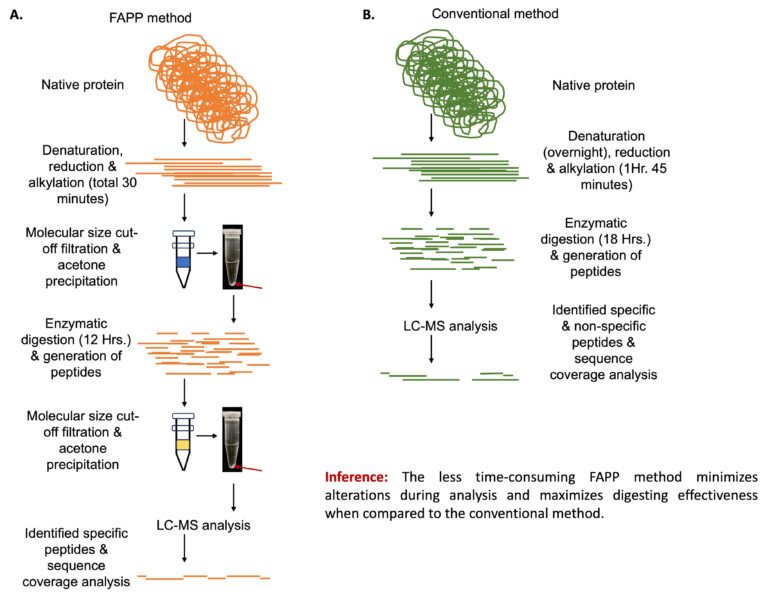
It could be challenging to prepare a bottom-up proteomic sample, digest it effectively, and then extract peptides for subsequent mass spectrometric analysis. The sample preparation for traditional protein digestion techniques requires highly trained professionals and still carries the risk of allowing chemicals that are required for extraction but likely to obstruct digestion, resulting in complex chromatographic profiles due of semi-cleavages, insufficient peptide cleavages, and other unwanted events. Additionally, peptide clean-up using frequently used immobilised C-18 pipette tips may cause significant peptide loss and individual peptide yield variability, resulting in artefacts of various product-related alterations. Therefore, we proposed an easy-to use, highly reproducible, appropriate and robust enzymatic protein digestion protocol for less trained professionals, that includes multiple filtration steps in between digestion processes, by various molecular weight filters, alongside protein precipitation, in order to minimise interference of necessary digestion chemicals. This efficiently overcomes the shortcomings of conventional digesting technology, boosts peptide yield, and removes the need for peptide clean-up.
The proposed method, which we termed “filter-assisted protein precipitation (FAPP),” allowed us to minimise digestion-related alterations and locate nearly all possible peptides with the site-specific modifications that retain great importance for biosimilarity assessment. Additionally, the thorough coverage of the CDR peptides greatly boosted confidence in the FAPP method’s capacity to confirm the primary structure of the under-researched biosimilars.
“This newly developed protocol will be an added advantage for the biopharmaceutical sector and impact biotherapeutic development to a great extent.”
How do these findings contribute to your research area?
This study introduces a novel sample preparation protein digestion method that is modest, highly reproducible, and far simpler to carry out without the assistance of a skilled professional. In our view, this newly developed protocol will be an added advantage for the biopharmaceutical sector and impact biotherapeutic development to a great extent.
What was the exciting moment during your research?
Creating a fully digested and more precise accurate peptide yield was the difficult part of our effort. Through chilled acetone precipitation following each filtration stage, we were able to do it over time. The achievement of this in a simple manner was therefore the most exciting moment for us.
What do you hope to do next?
We consider working towards the increasingly evident need for the development of new therapeutics as well as the novelty of the biotherapeutic
Where do you seek scientific inspiration from?
I can distinctly recall the life sciences classes I took in school under the tutelage of Mr. Debashish Chakraborty. His explanations of biological concepts motivated me to pursue further study in biological science. Additionally, my Ph.D. supervisor, Prof. Arnab Sen (NBU), Dr. Malay Bhattacharya (NBU) assisted me in tackling research challenges, and my present mentor, Prof. Anurag Singh Rathore (IITD), who continually inspires me to seek innovation and unconventional thinking in science.
How do you intend to help Indian science improve?
As a post-doctoral researcher, I aim to contribute to the cause of giving Indian science more exposure abroad and improving networking chances with foreign scientists. Additionally, a stronger outreach strategy for biopharmaceutical applications in the Indian market is another thing I want to contribute more to in the near future.
Reference: Bhattacharya S, Rathore AS. A novel filter-assisted protein precipitation (FAPP) based sample pre-treatment method for LC-MS peptide mapping for biosimilar characterization. Journal of Pharmaceutical and Biomedical Analysis. 2023 Jun 15:115527. https://doi.org/10.1016/j.jpba.2023.115527
Edited by: Anjali Mahilkar
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.