Hydrogel Chemo: Triple-Neg Breast Cancer
Work done in the lab of Prof. Ujjaini Dasgupta, Prof. Avinash Bajaj, and Dr. Arnab Mukhopadhyay at Amity University (Gurgaon), Regional Centre for Biotechnology (Faridabad), and National Institute of Immunology (New Delhi) respectively.
Animesh Kar is a highly qualified individual with a strong background in the field of biomedical science. He hails from Midnapore, West Bengal, and completed his undergraduate studies at the University of Delhi, earning a Bachelor’s degree in Biomedical Science. He then went on to earn a Masters’ degree in Biophysics from the All India Institute of Medical Sciences in New Delhi. In 2016, he began his Ph.D. studies under the guidance of Prof. Avinash Bajaj at the Regional Centre for Biotechnology in Faridabad and Prof. Ujjaini Dasgupta, Amity University Haryana. In addition to his work, Animesh has a passion for reading science fiction and non-fiction books, sketching scientific and non-scientific illustrations, and wildlife photography.

Dolly Jain has a strong background in biomedical science, having earned both a Bachelor’s degree and a Master’s degree in Biomedical Sciences at the University of Delhi. She also received the prestigious Hargobind Khorana Fellowship in 2018 where she did her internship in the lab of Daniel J. Klionsky at the University of Michigan and learned about autophagy and its deregulation in different pathologies. To further pursue her interests in cancer immunity, she started her Ph.D. work in 2020 under the guidance of Prof. Avinash Bajaj at the Regional Centre for Biotechnology in Faridabad. Her research work currently focusses on alterations in the immune microenvironment of cancer following different therapeutic interventions.

Sandeep Kumar earned a M.Sc. degree in Chemistry from Kurukshetra University, Haryana, and a PhD in Biotechnology under the direction of Prof. Avinash Bajaj from the Regional Centre for Biotechnology, Faridabad, India. His PhD research training centered around the chemical synthesis and preclinical evaluation of self-assembling biomaterials for biomedical applications. Currently, He is pursuing his postdoctoral research career at Johns Hopkins University School of Medicine, Baltimore, USA, and developing immunoengineered platforms to improve the efficacy of existing vaccine formulations against cancer and infectious agents.

Author Interview
How would you explain your research outcomes to the non-scientific community?
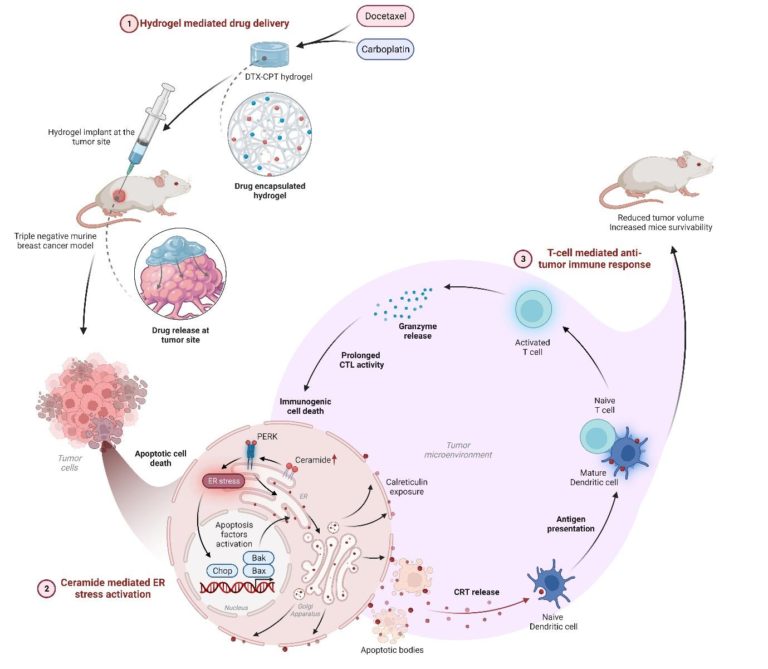
Our research focused on finding a new approach to treat Triple-Negative Breast Cancer (TNBC), the most aggressive breast cancer subtype, which is still a challenge. We developed a specialized gel that can deliver two chemotherapy drugs, docetaxel and carboplatin, directly to the tumor site. This localized delivery system has several advantages: it reduces the toxic effects of the drugs on healthy tissues and organs, enhances the anticancer effects, and stimulates the body’s immune response against the cancer cells.
Our findings demonstrated that this gel-based treatment was successful in different TNBC experimental models. It successfully altered the tumour microenvironment, which is normally immune-suppressed, by boosting the infiltration of anti-cancer immune cells. The gel induced the cancer cells to produce apoptosis-causing lipid molecules, which in turn boosted immunogenic cell death. These apoptotic cells then release chemicals that stimulate the immune system, allowing it to recognise and destroy cancer cells in the body, including distant metastatic tumours.
“Gel-based chemotherapy boosts immune response against aggressive breast cancer.”
How do these findings contribute to your research area?
These discoveries have a substantial impact on cancer therapy, especially for aggressive TNBC which is difficult to treat due to the absence of specific therapeutic target/s and the formation of a tumour immunosuppressive microenvironment (TIME). This study addresses these issues by providing a hydrogel-mediated localised delivery method for DTX-CPT-Gel treatment, a combination therapy of docetaxel (DTX) and carboplatin (CPT).
These findings advance our understanding of the interplay between chemotherapy, immunogenic cell death, and tumor microenvironment. By combining chemotherapy-induced cell death with immune modulation, the localised DTX-CPT-Gel treatment offers a potential method for effectively treating TNBC. It provides a valuable framework for future research and the development of innovative combination strategies for the treatment of TNBC and other types of cancer.
What was the exciting moment during your research?
Animesh Kar: DTX-CPT-Gel was hypothesized to regress tumor volume due to dual drug release at the tumor site. Significant regression in tumor volume and increased mice survivability strengthens our hypothesis. When we looked at the levels of various immune cells in the presence of DTX-CPT-Gel, we observed a major tumor microenvironment reprogramming that elicited an anti-tumor immune response.
Dolly Jain: We demonstrated effective inhibition of tumor growth by DTX-CPT-Gel. This led us to focus on modulation in the immune microenvironment and our observations suggested a shift towards increased T-cell immunity upon treatment with DTX-CPT-Gel therapy. Further mechanistic studies revealed that DTX-CPT-Gel alters tumor cell intrinsic mechanisms that are responsible for enhanced anti-tumor immunity and tumor growth inhibition.
Sandeep Kumar: Designing materials for drug delivery applications poses a significant challenge, particularly in terms of ensuring biocompatibility and safety. However, our hydrogel has demonstrated an exceptional safety profile in rigorous animal model testing. This exciting result has paved the way for further exploration of our hydrogel materials to address several clinical hurdles related to chemotherapy. One such challenge is the need for frequent administration of anticancer drugs to achieve complete tumor regression. By leveraging the unique properties of our hydrogel, we developed a drug delivery system that can release multiple anticancer drugs in a controlled manner, extending their efficacy and reducing the frequency of administration. This approach has the potential to improve patient outcomes and minimize the side effects associated with traditional chemotherapy regimens.
What do you hope to do next?
Further preclinical and clinical research would be required to verify the hydrogel-mediated delivery mechanism and examine the efficacy of the therapy and associated safety in human TNBC patients. These efforts will contribute to advancing the development of this promising treatment approach and providing a new therapeutic option for TNBC patients.
Where do you seek scientific inspiration from?
Animesh Kar: My interest in the sciences began when I first observed a plant cell under a microscope. This sparked a curiosity in me to understand the complex mechanisms that govern life. I find great pleasure and fulfillment in uncovering new information, solving unresolved questions, and developing solutions that can contribute to the advancement of humanity. The works of renowned planetary scientists and communicators, Neil DeGrasse Tyson and Sir David Attenborough, continue to inspire me in my passion for science. I am also deeply grateful for the guidance and inspiration provided by my guide, Prof. Avinash Bajaj, and co-guide, Prof. Ujjaini Dasgupta, who have encouraged me to take on new challenges and strive for excellence in scientific research.
Dolly Jain: As I was growing up, I saw many people around me having different ailments and disorders that affected them in different ways. Reading about the discoveries of the first antibiotic and first vaccine for infectious diseases has incited my interest in this field of studying different diseases and finding cures that can help individuals fight these ailments. What amazed me the most were the immune cells whose sole purpose is to protect the body from ailments untill they die. I feel satisfaction in studying the role of immune cells in diseased conditions and how we can alter them to cure these conditions, particularly cancer. My guide, Prof. Avinash Bajaj, and mentor, Prof. Ujjaini Dasgupta, gave me this opportunity to pursue my interests and provided constant encouragement to follow this passion.
Sandeep Kumar: My research journey began with a profound fascination for organic chemistry, particularly synthetic chemistry, exploring novel chemical reactions and compound design. Identifying the transformative potential of organic chemistry in addressing pressing clinical challenges, such as the need for superior antimicrobials, development of advanced drug discovery methods, and strategies to improve patient outcomes. I find motivation in identifying the potential applications of chemistry in medicine and acquiring knowledge from diverse areas of human disease biology through interdisciplinary approaches.
How do you intend to help Indian science improve?
Our study has the potential to significantly contribute to the advancement of Indian science in a variety of ways. To begin, TNBC therapy is extremely difficult, and it affects a disproportionately large percentage of breast cancer patients in India. Our study presents a treatment strategy for TNBC that has the potential to improve anticancer efficacy while reducing systemic toxicity, addressing the key issue of side effects associated with existing chemotherapy regimens. The localized delivery system also presents the potential to clear distant and metastatic tumors, which can significantly improve the overall survival rates of Indian TNBC patients. Furthermore, our study sheds light on the modulation of the TIME in TNBC and provides scope for exploring combination chemotherapy with immunotherapy. This immune modulation aspect of our research has implications beyond TNBC and can contribute to the broader field of cancer immunotherapy, including various kinds of cancer prevalent in the Indian population. Overall, by improving treatment outcomes and minimizing toxicity, our approach has the potential to enhance the standard of care for TNBC patients in India.
Reference:
Animesh Kar, et al., A localized hydrogel-mediated chemotherapy causes immunogenic cell death via activation of ceramide-mediated unfolded protein response. Sci. Adv. 9, eadf2746 (2023). DOI: 10.1126/sciadv.adf2746
Edited by: Sukanya Madhwal
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.