NanoCure: siRNA + Lipopeptide Combo for Breast Cancer
Work done in the lab of lab of Dr. Rituparna Sinha Roy at IISER Kolkata and collaborator Arnab Mukherjee – IISER Pune
Argha Mario Mallick is a PhD student in Dr. Rituparna Sinha Roy’s lab, Department of Biological Sciences, IISER Kolkata. Argha Mario Mallick completed his B.Sc. in Zoology (Hons) from Krishnagar Govt College and M.Sc. in zoology with specialization in Human genetics and molecular biology from The University of Burdwan. He works in developing peptide-based combination therapy against cancer.

Author Interview
How would you explain your research outcomes to the non-scientific community?
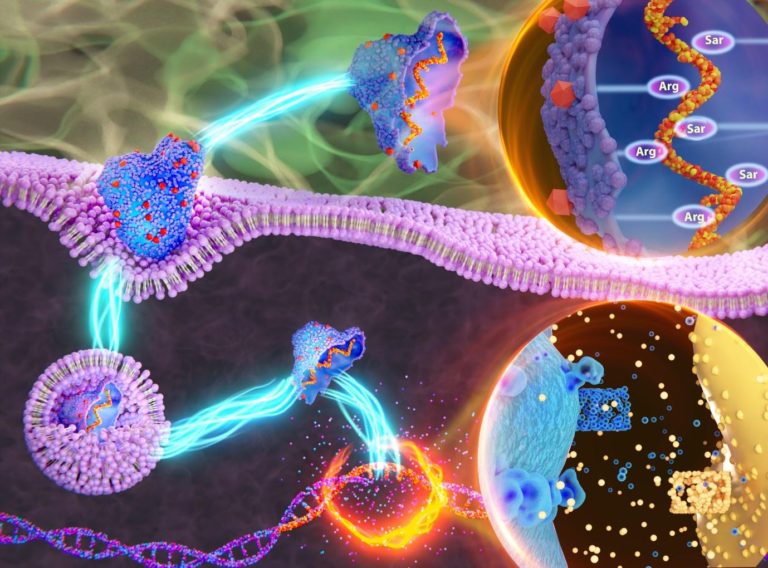
Cancer mostly occurs when certain genes(oncogenes) are overproduced (overexpressed). Small interfering RNA (siRNA) which has a sequence complementary to these oncogenes can bind to these oncogenes and degrade them. siRNA is easy to design and synthesize for particular oncogenes, so theoretically, siRNA should destroy genes responsible for cancer progression. However, siRNA is very unstable and the main challenge is to deliver this siRNA safely inside the cell (cytoplasm) as there are many physiological barriers for a siRNA to reach the cytoplasm. siRNA-based treatment got FDA approval in 2018. siRNA alone cannot enter cells and has to be conjugated with delivery agents. The existing delivery systems for siRNA are viral and lipid-based which are toxic and can destroy genes non-specifically, i.e., destroy other genes instead of or in addition to destroying the desired oncogene. To solve this problem, we have designed a peptide-based delivery system that is completely non-toxic to cells. In addition to being non toxic to normal human cells our siRNA delivery system can destroy oncogenes with the same efficacy as done by highly costly siRNA delivery agents sold by multinational companies, but which are toxic to normal cells. Our delivery system has vitamin E attached to it. Vitamin E is known to scavenge ROS (reactive oxygen species) which causes cancer. Thus, our delivery system also has a chemo-preventive role against cancer. Our delivery system has an alternating Arginine- Sarcosine-Argine backbone which makes its optimum protease stable. Optimum protease stability of the delivery system ensures enhanced cytosolic delivery of siRNA without being bio-accumulated in the living system and also ensures the decomplexation of siRNA from the peptide in the cytoplasm which is critically needed for gene silencing.. The synthesized nano-complex can escape both early and late endosomes. Our delivery system has silenced oncogene notch1 oncogene in human triple-negative breast cancer (one of the hardest to treat form of breast cancer with a high mortality rate, prevalent in the young Indian population) cells with the same efficacy as that of HiPerFect (a commercially used siRNA delivery system sold by multinational company Qiagen, Germany). Notch1 oncogene is responsible for chemoresistance and high rate of metastasis (spreading of cancer cell to different organs) of triple negative breast cancer. Some cells like primary cells (which can generate different types of cells) are especially difficult to internalize siRNA inside them and are known as “hard to transfect” cell lines. Our synthesized delivery system internalized siRNA in “hard to transfect” HUVEC (cells isolated from the human umbilical cord) 1.53 times more than HiPerFect. Moreover, our designed delivery system provides longer-term oncogene silencing than HiPerFect. On day 7, after treatment, our synthesized delivery system silenced oncogene notch1 of human triple-negative breast cancer cells 1.6 times more than HiPerFect. Recently it has been observed that the nanobridges are formed from triple-negative breast cancer cells to endothelial cells. Transfer of miRNA occurs and this makes the endothelial cells pathogenic and aids in metastasis. Our engineered nano complexes can also prevent these nanobridge formation as well.
We have also shown that our synthesized nano complex can augment its therapeutic efficacy against human triple-negative breast cancer cells when used in combination with the commonly used anti-diabetic drug metformin, re-purposed for cancer therapy. These drugs in combination exhibited synergistic effects (better effect if used together than separately) against human triple-negative breast cancer cells. This combinatorial regimen further lowered metastasis and stemness (property to generate more cancer cells) of human triple-negative breast cancer cells. We have shown cancer cell proliferation and metastasis have highly diminished by our proposed combination therapy in the zebrafish animal model. Our designed delivery system is non-immunogenic i.e., does not elicit any abrupt immunogenic response, which is a very important criterion for developing peptide-based drugs.
How would you explain your research outcomes for readers having basic scientific knowledge?
RNAi technology is an emerging molecular therapeutic platform for treating various incurable diseases having undruggable targets, including cancers and rare genetic disorders. FDA approved the first RNAi therapy (Patisiran) in 2018. siRNA has been instrumental in achieving the therapeutic potential of RNAi by theoretically silencing any gene of interest in a sequence-specific manner. The main challenge lies in the cytoplasmic delivery of siRNA. The existing, widely used lipid-based siRNA delivery vehicles are highly toxic to cells and are reported to induce their gene signature to the cargo nucleic acid and increase non-specific gene silencing. Towards this goal, we have engineered clinically safe, non-immunogenic peptide-based siRNA delivery vehicles, which can form self-assembled nanocomplex with siRNA at room temperature and exhibit high gene knockdown efficacy.
The engineered peptide-based siRNA delivery vehicle has an alternating Arg-Sar-Arg backbone. The incorporation of sarcosine as a spacer in between two arginine residues minimizes the adjacent Arg-Arg repulsion and provides optimum proteolytic stability to the peptide. Optimum proteolytic stability confirms enhanced cytosolic delivery of siRNA, the delivery vehicles are not bio-accumulated in the living system and also ensures the decomplexation of siRNA from the peptide in the cytoplasm for accessing the RISC complex. We have incorporated a long partially unsaturated lipidic moiety of vitamin E and a short saturated lipidic moiety (octyl chain) into our peptide-based siRNA delivery vehicle. Unsaturation in lipidic moiety and shorter alkyl chain ensure weaker association of the delivery vehicle with the cell membrane and endosomal membrane and thus facilitate cytosolic internalization. Vitamin E lipidic moiety is chosen to gain swift FDA approval for our molecule. The designed delivery vehicles are non-toxic to both normal human cells and human cancer cells compared to HiPerFect (transfection reagent sold by multinational company Qiagen) which exhibits significant toxicity. Sarcosine residues provide protease stability to our designed peptide and optimum protease stability of siRNA transporter could protect siRNA in the presence of serum and RNase A. Our designed siRNA transporter internalized siRNA into the cytoplasm of both the human triple-negative breast cancer cell line and the “hard to transfect” primary cell line HUVEC significantly higher than that of HiPerFect. These nano complexes can also escape from both early and late endosomes. Please note that it is advantageous if siRNA can escape from the early endosome, which is beneficial for its functionality. Well-known TAT peptides can escape only from late endosomes. Having all the characteristics of an ideal siRNA transporter, our designed nano complexes can silence Notch1, Erk1 and Erk2 genes in human triple-negative breast cancer cell lines both at gene and protein level with comparable efficacy to that of HiPerFect. Interestingly, our designed nano complex exhibited longer-term gene knockdown than that by HiPerFect. On day 7, our engineered nanocomplex demonstrated 1.6 times higher Notch1 gene silencing compared to that by HiPerFect. Silencing Notch1 in human triple-negative breast cancer cell lines by our synthesized nano complex also reduced metastasis and stem cell markers. Moreover, this nano complex could prevent the formation of nano-bridges connecting cancer cells and endothelial cells, which makes the endothelial cells pathogenic and aid in the metastasis of cancer cells. We have also shown that the designed Notch1 silencing nano complex exhibits synergistic drug interaction with anti-diabetic, anti-aging repurposed drug metformin in human triple-negative breast cancer cell line and this combination therapy, further prevents proliferation, and micro metastasis of cancer cells than only Notch1 silencing in in vivo model. We have shown that our siRNA transporter is non-immunogenic which is a very important criterion for developing peptide-based drugs.
“We have synthesized a short peptide-based non-immunogenic, clinically safe, siRNA delivery vehicle which is cost-effective and non-toxic compared to lipid-based transfection reagent sold by multinational companies which exhibits significant toxicity.”
How do these findings contribute to your research area?
We have synthesized a short peptide-based non-immunogenic, clinically safe, siRNA delivery vehicle which is cost-effective and non-toxic compared to lipid-based transfection reagent sold by multinational companies which exhibits significant toxicity. These engineered lipopeptides may have a bright prospect as delivery agents for developing mRNA vaccines, gene editing, and reprogramming cancer cells.
What was the exciting moment during your research?
The first time I saw that our designed siRNA transporters could silence desired oncogenes with the same efficacy as done by HiPerFect but with long-term gene silencing efficacy. I was also excited when the common anti-diabetic drug exhibited synergistic drug interaction with our designed notch1 silencing nanoparticles and improved the therapeutic outcome both in in vitro and in vivo conditions.
What do you hope to do next?
I hope to design further peptide-based therapeutics for other hard-to-treat types of cancers.
Where do you seek scientific inspiration from?
The common people of India. Visit to any government hospital motivates me to pursue science. Visit to Tata Memorial Hospital, Mumbai before joining my Ph.D. had a great impact on me. Stories of struggle of some very sharp minds (my friends at the hostel) have kept me motivated during my Ph.D. tenure.
How do you intend to help Indian science improve?
I wish to help in developing cost-effective healthcare materials. I think the collaborative work of academia with industries can be a great boon for Indian science. I wish to remain in the field of translational science and academia to inspire young Indian minds in the future.
Reference:
M. Mallick, A. Biswas, S. Mishra, S. Jadhav, K. Chakraborty, A. Tripathi, A. Mukherjee and R. S. Roy. Engineered vitamin E-tethered non-immunogenic facial lipopeptide for developing improved siRNA based combination therapy against metastatic breast cancer, Chemical Science, 2023. DOI: 10.1039/D3SC01071F
Edited by: Ritvi Shah
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.