Novel Neuronal Function: IP3R's Role Beyond Channels
Work done in the lab of Prof. Gaiti Hasan at National Centre for Biological Sciences, TIFR, Bengaluru.
Pragnya Chakraborty holds Bachelor’s and Master’s degrees in Pharmacy from Jadavpur University, Kolkata. Driven by her passion for neuroscience, she pursued her doctoral degree in Prof. Gaiti Hasan’s Lab at the National Centre for Biological Sciences, TIFR, Bangalore. Her research centered on the IP3 receptor’s role in neuronal store-operated calcium entry using human neural stem cells and neurons. Pragnya collaborated closely with Prof. Colin W. Taylor at the University of Cambridge, UK. Having completed her Ph.D. journey at NCBS, she is currently working as a postdoctoral fellow at SickKids Hospital, University of Toronto, Canada.
Author Interview
How would you explain your research outcomes to the non-scientific community?
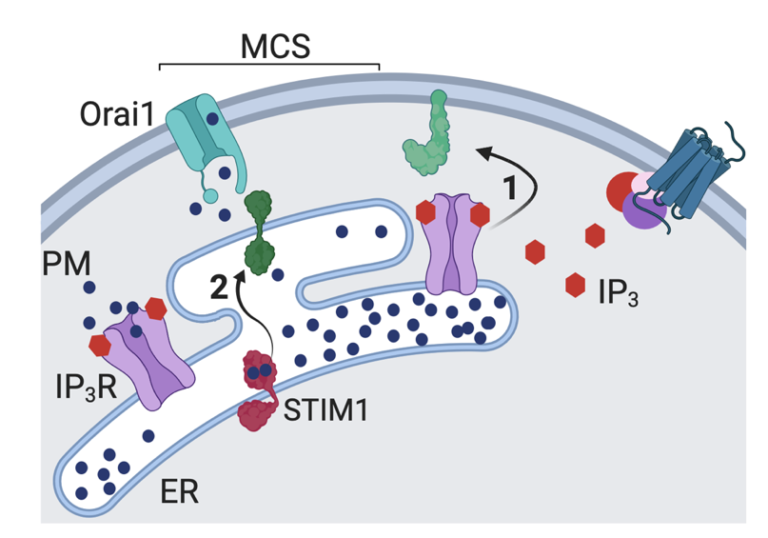
The synchronized brain function in any living being hinges on intensive neuronal communication. Serving as a secondary messenger within our nerve cells or neurons, calcium (Ca2+) plays a vital role in transporting and facilitating crucial cellular signals apart from managing several other crucial cellular aspects. Therefore, maintaining proper Ca2+ concentration in and out of neurons is absolutely essential for normal brain function. To save the cells from calcium overload or toxicity, there are “mini-warehouses” (intracellular organelles) to store the excess Ca2+ known as “intracellular Ca2+ store”. The endoplasmic reticulum (ER) is the largest intracellular organelle and houses the maximum amount of Ca2+. When the cell gets a signal (Receptor activation-induced IP3 production in the cytosol), it can open the door known as the IP3 receptor (inositol 1,4,5-triphosphate receptor or IP3-gated Ca2+ channel, IP3R) on the ER membrane and release some of the stored calcium. Such drop in Ca2+ levels inside ER is sensed by a ER resident sensor protein known as “STIM” which activates a process that allows Ca2+ influx from the outside of the neurons to come in through a specialised Ca2+ selective channel known as “Orai”. This calcium store-driven mechanism of Ca2+ influx is known as store-operated Ca2+ entry (SOCE). In this paper, we have demonstrated a novel mechanism by which the IP3 receptor regulates neuronal SOCE. In human neurons, SOCE evoked by pharmacological depletion of ER-Ca2+ is attenuated by loss of IP3Rs, and restored by expression of IP3Rs even when they cannot release Ca2+, but only if the IP3Rs can bind to its ligand IP3. Imaging studies demonstrate that IP3Rs enhance the association of STIM with Orai in neuronal cells with empty stores; this requires an IP3-binding site, but not a channel.
“Therapeutic strategies that target neuronal regulators of SOCE may be of value in multiple such neurodegenerative conditions. “
How do these findings contribute to your research area?
The close connection between the IP3 receptors and SOCE in neurons raises questions as to the extent to which SOCE contributes to neuronal phenotypes attributed so far to reduced activity of the IP3R alone. These include neurological diseases like spinocerebellar ataxia 15 (SCA15), spinocerebellar ataxia 29 (SCA29), Parkinson’s disease, Alzheimer’s disease, Huntington’s and Gillespie’s Syndrome. The extent to which such disease-causing mutations, specifically mutations of the IP3-binding domain of the IP3R1, impact neuronal SOCE needs investigation. Therapeutic strategies that target neuronal regulators of SOCE may be of value in multiple such neurodegenerative conditions. This paper will capture the interest of scientists studying cell biology or neurobiology, specifically those interested in calcium signaling pathways, particularly store-operated calcium entry.
What was the exciting moment during your research?
A previous paper from our lab had already documented such IP3R-mediated SOCE regulation in Drosophila neurons (Link), but the molecular mechanism was still unclear. I was curious to know whether such regulation is also present in human neurons. Among numerous thrilling instances, the memory of observing the phenotype for the very first time in human neural stem cells during calcium imaging stands out as an everlasting moment that I will cherish forever.
Where do you seek scientific inspiration from?
Nature acts as a rich source of inspiration, as observing natural occurrences has historically raised inquiries that have propelled fundamental research endeavors. These foundational basic research works in turn lay the groundwork for later breakthroughs and translational advancements. Overall, the pursuit of knowledge, the thrill of discovery, and the desire to contribute to our understanding of the world are constant sources of inspiration in my scientific journey. The joy of knowing and observing something for the first time that nobody else knows on the entire planet is absolutely surreal.
How do you intend to help Indian science improve?
I aim to enhance Indian science by conducting impactful research, fostering interdisciplinary collaborations, and promoting science communication. By fostering interdisciplinary interactions I can help to bridge the gap between laboratory research and practical applications, leading to meaningful contributions, I aspire to contribute to scientific advancements and inspire future generations of scientists.
Reference:
Pragnya Chakraborty Bipan Kumar Deb Vikas Arige Thasneem Musthafa Sundeep Malik David I Yule Colin W Taylor Gaiti Hasan. (2023) Regulation of store-operated Ca2+ entry by IP3 receptors independent of their ability to release Ca2+.
eLife 12:e80447.
https://doi.org/10.7554/eLife.80447
Edited by: Anjali Mahilkar
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.

