Mechanical roles of structurally different osmolytes with similar molecular mechanism
Work done in the lab of Dr. Shubhasis Haldar at Structural Mechanobiology Lab, SN Bose National Centre for Basic Sciences, Kolkata
Deep Chaudhuri is a doctoral fellow doing research on the single-molecule biophysics field in Structural mechanobiology lab under the guidance of Dr. Shubhasis Haldar. His research interests lie at the intersection of physics, chemistry, and biology. Currently, he is investigating the mechanical roles of bacterial chaperones under single-molecule force spectroscopic technique. He is keenly interested in deciphering the mechanism governing the mechanical stability of the protein across various cellular processes and wants to investigate the holistic picture of cellular mechano-transduction events.
Author Interview
How would you explain your research outcomes to the non-scientific community?
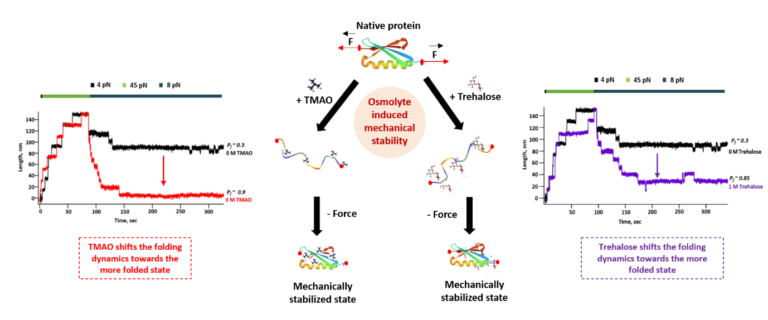
Osmolytes are a kind of small molecule known to be found in cells, playing a crucial role in maintaining the structural stability of the protein molecule in the challenging environments. They are basically molecular caretakers that help to protect the protein molecules in different temperatures, pressure or in higher salt concentrations. Some osmolytes like TMAO glycerol help to stabilize the protein structure, whereas other osmolytes like trehalose stabilize the unfolded state of their protein molecule. Despite their stabilizing effect, they can also interact with the protein molecules with various mechanisms like preferential exclusion of water bodies or binding to the transition state of protein (a fleeting state between native and unfolded structure). Since mechanical stability of the protein is an indispensable aspect for the characterising of their folding phenomena, we wanted to understand the detailed molecular mechanism of the protein-osmolytes interaction by investigating the change in the folding dynamics of the substrate protein at equilibrium condition.
We have developed one of the most advanced single-molecule force spectroscopic technique covalent magnetic tweezers in our lab to understand the folding dynamics of a single protein molecule in the presence of two structurally different osmolytes TMAO and trehalose. We have found that both these osmolytes increase the mechanical stability of the substrate protein L by increasing the mechanical stability by increasing the unfolding force up to 1.3 folds and tilting the energy landscape towards the more folded state. We found that these osmolytes accelerate refolding and retard the unfolding of the substrate protein that promotes to stabilize the protein molecule at its folded state. We have explained the underlying molecular mechanism of protein L-osmolyte interaction with steered molecular dynamic simulation. This result explains that the osmolytes destabilized the unfolded state of the protein by disrupting the solvent hydrogen bonding. Overall, our combining single-molecule force spectroscopic study and advanced steered molecular dynamic simulation helps to explain the in-depth molecular mechanism of the protein in the presence of two structurally different osmolytes.
“Our findings shed light on how osmolytes can modulate protein stability, offering insights into strategies for engineering proteins with desired folding kinetics and structural properties. “
How do these findings contribute to your research area?
Understanding the molecular mechanisms of underlying protein folding and stability is crucial for developing therapeutics and biotechnological applications. High concentration of osmolytes (like TMAO) are associated with various human diseases, such as hypertension, atherosclerosis and multiple cardiovascular disease. Our findings shed light on how osmolytes can modulate protein stability, offering insights into strategies for engineering proteins with desired folding kinetics and structural properties. Additionally, this result can also contribute to a broader understanding of how environmental factors influence protein behaviour, which is essential for designing drugs associated with protein conformational disease and optimizing the mechanism of various cardiovascular diseases.
What was the exciting moment during your research?
As a student with a background in Chemistry, I have always found biological experiments interesting, offering a fresh perspective on science. However, conducting single-molecule experiments always presents unique challenges and obtaining results can be very exciting. In this project one particularly exciting moment during my research was when I observed the significant increase in protein L unfolding force by up to 1.3-folds in the presence of osmolytes, especially at 3M TMAO. This observation provided a clear indication of the mechanical stability enhancement conferred by osmolytes, shedding light on their role in modulating protein folding under mechanical force. These results not only validated our hypotheses but also opened new avenues for understanding and manipulating protein stability for various biotechnological applications.
What do you hope to do next?
Upon completing my Ph.D. I want to explore more in the single-molecule biophysics field through a postdoctoral position. Additionally, I also aspire to contribute my experiences and thoughts to the academic administrative sector.
Where do you seek scientific inspiration from?
In the single-molecule biophysics field, the fascination lies in observing the intricate behaviours and interactions at single-molecule level from a single experiment. Apart from that, the guidance and mentorship of our Ph.D. advisor was highly motivating. Alongside, the unwavering constant support provided by my fellow lab members, particularly our experienced seniors, in every aspect of my research journey served as a constant source of inspiration.
How do you intend to help Indian science improve?
As an interdisciplinary researcher I always want to promote the importance and significance of interdisciplinary research that leads to novel discoveries and solutions. Secondly, it’s very important to communicate the science aimed at engaging common public and policy makers for the awareness about the importance of scientific research and its social impact which can play an important role in the continuous growth and excellence of Indian science.
Reference: Chaudhuri D, Chowdhury D, Chakraborty S, Bhatt M, Chowdhury R, Dutta A, Mistry A, Haldar S. Structurally different chemical chaperones show similar mechanical roles with independent molecular mechanisms. Nanoscale. 2024 Feb 1;16(5):2540-2551. https://pubs.rsc.org/en/content/articlelanding/2024/nr/d3nr00398a
Biopatrika: Bringing Science to Society
© Biopatrika 2024 All Rights Reserved.
