Nature’s seed secret: Elusive plant amyloids as facilitators of seed germination
Work done in the lab of Prof. Ashwani Kumar Thakur at Department of Biological Sciences and Bioengineering, Indian Institute of Technology Kanpur.
Nabodita Sinha has completed her Bachelor’s and Master’s in Science in Biotechnology from Maulana Abul Kalam Azad University of Technology, with a First Class First Gold medal award for both degrees. She joined the Indian Institute of Technology Kanpur in 2017 to pursue a doctoral degree. During this period, her independent projects led to an award of MTech Degree as well. She is the recipient of multiple awards including Nikon Photomicrography competition, Carl Storm International Diversity Fellowship for Oral Presentation at the Gordon Research Conference and was invited as Session Chair in Botany-2022 Conference in Alaska (Hybrid mode). She was also awarded with the prestigious EMBO Short-term Scientific Exchange Grant for pursuing research at the University of Tokyo, Japan for three months.
Author Interview
How would you explain your research outcomes to the non-scientific community?
Proteins are one of the major biomolecules for providing structure and nutrition to any life form. However, under certain aberrant conditions or to perform specialized functions, some proteins might aggregate and form structures called amyloids. These amyloid structures are much more resilient to environmental perturbations than normal proteins as they are formed by layering and twisting of individual proteins. Amyloids are involved on one hand in multiple pathological conditions including diabetes, cataract, and neurodegenerative diseases including Alzheimer’s. On the other hand, they also perform functional roles, for example melanin synthesis in humans. These amyloids were known to be present in almost every kingdom of life except plants. Thus, it was surprising that despite the conservation of amyloids in almost all types of species, these remained elusive in the plants.
On searching the scientific literature, we found similarities (size, shape and appearance) between plant seed protein storage compartments (seed storage protein bodies) and amyloid-containing protein bodies of other organisms (bacterial inclusion bodies for example), and thus proposed that seed storage protein bodies (SSPB) might contain amyloid structures.
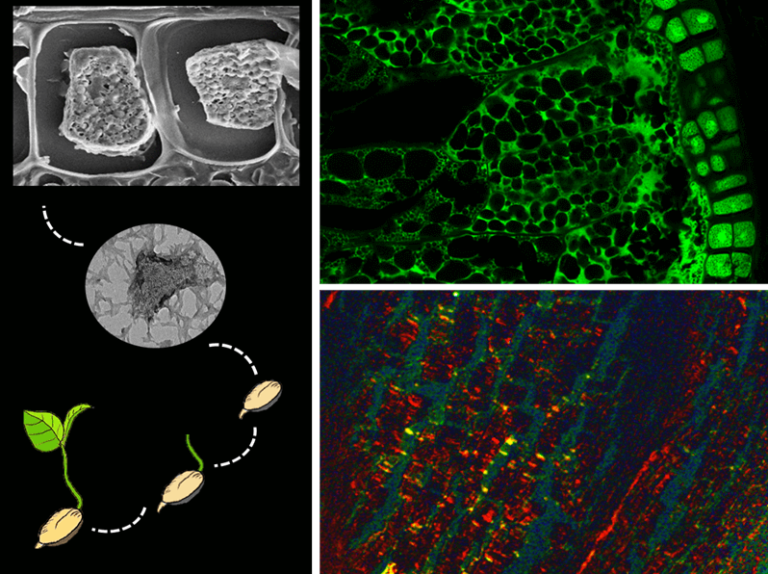
In this study, we have first employed multiple amyloid-specific dyes (ThT, Proteostat, and Congo red) that are used to detect amyloids in any cell or tissue. All the amyloid-specific probes showed a positive signal of amyloid structures in the cereal (wheat, barley) and pulse (mungbean, chickpea) seeds. Among these dyes, Congo red (CR) is known as the “gold-standard” probe for detecting and confirming amyloids in human and animal tissues. However, CR also binds to the plant cell walls and can yield similar signals to amyloids. To remove the ambiguity of staining among proteinaceous amyloids and cellulose-like carbohydrates, we digested the plant cell walls and isolated protoplasts (living plant cells without cell walls). The protoplasts also exhibited amyloid signatures with all the dyes, proving the presence of proteinaceous amyloids. The isolated SSPB similarly showed amyloid-signatures and confirmed amyloid presence in plants. We next dissected the amyloid areas, specifically from the seed tissues, using a high-precision laser and performed mass spectrometry to identify the amyloid-forming proteins. It was found that the seed globulin proteins are the major proteins that form amyloids.
The next question was why nature has provided amyloids in plants. To establish this, we allowed seed germination and seedling growth and checked the pattern of total protein vs amyloid degradation throughout germination. We found that the total proteins remain constant throughout germination; the less-stable amyloid-like structures degrade fast, whereas the more stable amyloid structures degrade slower, maintaining a sustained degradation. Further, we confirmed that the stepwise degradation of the amyloids plays a critical role in maintaining the optimum germination index and is regulated by plant germination/dormancy inducing hormones (gibberellic acid, abscisic acid) and proteases.
Thus, we have proved in the current study for the first time that plant seeds contain amyloid structures, and these play a critical role in seed germination and seedling growth.
“The findings, for the first time, establish the presence of amyloids in the plant kingdom.”
How do these findings contribute to your research area?
The findings, for the first time, establish the presence of amyloids in the plant kingdom and hence open multiple research avenues for exploring the natural history of amyloid formation of SSPB, the role of seed amyloids in stress adaptation, and the potential of controlling amyloid content for better seed quality yield. Simultaneously, the study would incite the usage of high-resolution ultrastructural analysis of the SSPB amyloids and its molecular-to-atomic structural model building. Additionally, the seed amyloids might serve as an exciting candidate as biomaterials for controlled drug release.
What was the exciting moment during your research?
Due to the unexplored thematic area of the research and the novelty, we were fascinated and excited by each and every result, including the beautiful stained images of the amyloids, and their fibrillar structures in the electron microscopy. The most intriguing and exciting part of the whole journey for me was establishing not only the presence but also the functional aspects of the amyloids in the seeds for the first time. The second determining aspect was optimizing the laser capture microdissection and mass spectrometry to specifically identify the amyloid-forming globulin proteins. At each step, I was constantly supported by my supervisor and the amazing team at IIT Kanpur and Tata Translational Cancer Research Centre. The latest exciting moment of the research was when our article was chosen by the editor as a “key article” and was highlighted using a short “Research highlight”.
What do you hope to do next?
I would like to advance the ultrastructural analysis of the SSPB amyloids further for the time being. After my PhD, I would like to join the research and development sector of biopharmaceutical industries to deliver real-time solutions in this area.
Where do you seek scientific inspiration from?
For me, scientific inspiration comes from communication. When I pitch my research findings in oral or written form, I gain novel insights from this discussion as well as the audience, which drives further my scientific aspirations.
How do you intend to help Indian science improve?
I intend to continuously contribute to Indian science by utilizing the gained knowledge for writing editorials, scientific communications and for developing an integrated approach for managing strategies in the biopharmaceutical sector.
Reference:
Sinha N, Zahra T, Gahane AY, Rout B, Bhattacharya A, Basu S, et al. Protein reservoirs of seeds are amyloid composites employed differentially for germination and seedling emergence. Plant J. 2023;116(2):329-46.
Research Article link – https://onlinelibrary.wiley.com/doi/10.1111/tpj.16429
Research Highlight ink – https://doi.org/10.1111/tpj.16485
Edited by: Nikita Nimbark
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.

