Single-molecule FRET illuminates crucial molecular events of biological phase separation
Work done in the lab of Prof. Samrat Mukhopadhyay at the Indian Institute of Science Education and Research Mohali (IISER Mohali)
Ashish Joshi was born and brought up in Delhi, India. After schooling at a government school, Rajkiya Pratibha Vikas Vidyalaya, Kishan Ganj, Delhi, he joined Guru Gobind Singh Indraprastha University (GGSIPU) for his B.Tech in Biotechnology. He obtained his first research exposure during his B.Tech thesis project under the guidance of Professor Nimisha Sharma at GGSIPU. In 2018, Ashish Joshi was selected for the Ph.D. program at the Department of Biological Sciences at IISER Mohali under the supervision of Professor Samrat Mukhopadhyay. In the Mukhopadhyay lab, he is interested in understanding the molecular drivers of biological phase transitions associated with cellular physiology and pathology. He draws inspiration from his supervisor, Samrat Mukhopadhyay, and the late Dr. APJ Abdul Kalam in every aspect of life. Besides his scientific endeavors, he likes spending time with family, interacting with new people, and biking for a quick cup of tea.
Author Interview
How would you explain your research outcomes to the non-scientific community?
Living cells are a complex mixture of thousands of biomolecules, which participate in diverse chemical reactions within the controlled environment of various membrane-bounded organelles like endoplasmic reticulum, Golgi complex, mitochondria, and so on. Furthermore, cells employ a range of dynamic membrane-less organelles (MLOs) formed via the process of liquid-liquid phase separation, to achieve cytoplasmic compartmentalization. These protein and nucleic acids enriched MLOs or biomolecular condensates, which include nucleolus, stress granules, and P-bodies, etc., are responsible for some crucial cellular functions and are characterized by their liquid-like, dynamic, reversible nature. Extensive studies on these condensates have identified a class of proteins termed intrinsically disordered proteins/regions (IDPs/IDRs) as the primary drivers of these cellular condensates. These proteins lack a defined three-dimensional structure and exhibit large conformational heterogeneity, enabling large-scale networks of intermolecular interactions, and engendering the formation of an extensive mesh of protein molecules. This network of biomolecules is highly dynamic, resulting from the constant making and breaking of the ephemeral intermolecular contacts, and thus can easily be modulated by various cytoplasmic cues within the cells.
Hence, these cellular assemblies are instrumental in a plethora of physiological processes within the prokaryotic and eukaryotic cells. However, the formation and dysregulation of these condensates are also associated with several pathological aspects of the cells. Insoluble deposits and aggregates of these IDPs/IDRs within neuronal cells are implicated in a range of debilitating neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD), and so on. Biological phase transition is a relatively young field, and the underlying principles governing these phase transitions remain poorly understood. Hence, discerning the sequence of events and molecular mechanism modulating these pathophysiological phase transitions has rapidly gained significance in the path of understanding the molecular aspects of neurodegeneration.
Our work focuses on the prion-like intrinsically disordered low complexity region of Fused in Sarcoma (FUS-LC), a protein implicated in the pathology of ALS and FTLD. FUS-LC is a model IDP with a low-complexity amino acid sequence, rich in serine, tyrosine, glycine, and glutamine residues, and is believed to drive the phase transition of FUS. Using a highly sensitive, state-of-the-art technique, namely Förster Resonance energy transfer (FRET), in addition to other fluorescence and vibrational tools at the single-molecule level, we study the protein chain in the monomeric and the droplet phases and capture the changes in the polypeptide chain accompanying phase separation. Our results reveal the extensive conformational heterogeneity and the co-existing subpopulations of the FUS-LC polypeptide chain in both the dispersed and condensed phases, in addition to an unwinding of the polypeptide chain upon phase separation. The introduction of a disease-associated mutation resulted in further expansion of the FUS-LC polypeptide chain in the condensed phase. We hypothesize that this unraveling of the polypeptide chain leads to an enhanced multivalency, promoting a transition from intra-molecular to intermolecular contacts, which can further facilitate an accelerated aggregation of the mutant FUS-LC. This conformational unwinding, leading to the formation of a dense protein network, can be a general phenomenon in the phase separation of other IDPs/IDRs.
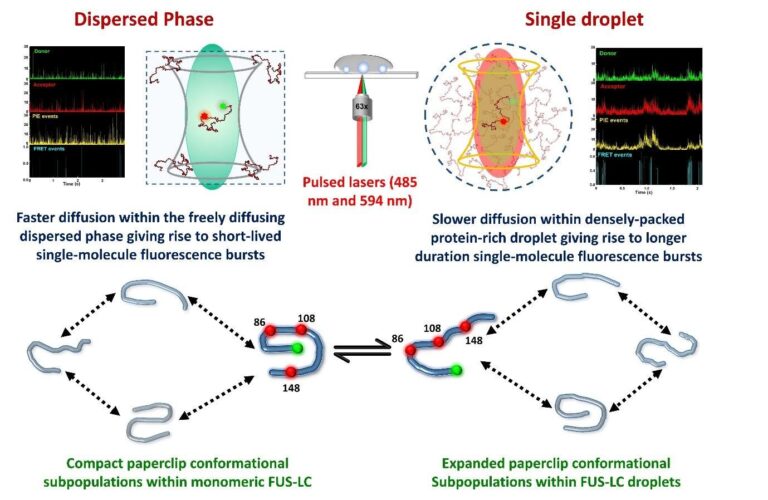
“Our work unveils the molecular events driving phase separation of an archetypical prion-like low-complexity domain of a protein known to undergo biological phase transitions with physiological and neuropathological functions.”
How do these findings contribute to your research area?
Our work unveils the molecular events driving phase separation of an archetypical prion-like low-complexity domain of a protein known to undergo biological phase transitions with physiological and neuropathological functions. These results can be further extended for other prion-like low-complexity domain-containing proteins involved in physiology and neurodegenerative diseases. We demonstrate the application of an ultra-sensitive tool, single-molecule FRET, in conjunction with other microscopic and spectroscopic tools in a droplet-by-droplet manner to obtain structural information at the molecular level. Based on these methodologies, the effect of phase separation modulators, including nucleic acids, post-translation modification, and disease-associated mutations, can be interrogated in-depth to shed light on the mechanistic underpinnings of biological phase transitions.
What was the exciting moment during your research?
This journey, as a whole, has been full of excitement, beginning with the acceptance of the grant for this state-of-the-art equipment, the installation training, and then obtaining the first single-molecule fluorescence bursts. Another exciting moment was obtaining these single-molecule fluorescence bursts within individual phase-separated condensates for the first time. Moreover, we could capture the expansion of the polypeptide chain from the dispersed to the condensed phase, facilitating phase separation.
What do you hope to do next?
During my PhD tenure, I learned various biophysical tools such as atomic force microscopy, super-resolution microscopy, steady-state and time-resolved fluorescence spectroscopy, vibrational Raman spectroscopy, and single-molecule fluorescence techniques. After my PhD, I wish to pursue my postdoctoral studies in the field of single-molecule biology. I want to explore further the structure-function relationships within the intricate biological systems at the single-molecule level.
Where do you seek scientific inspiration from?
My primary inspiration comes from the fact that lights will guide you to the right path if you have started something for a noble cause. Apart from that, I get genuinely motivated by seeing an extraordinarily hardworking Ph.D. advisor mentoring me and highly motivated students in my lab. Last but not least, I am inspired by some great sports people like Virat Kohli and Mary Kom, who have a never-say-die attitude even during the most challenging time of their career.
How do you intend to help Indian science improve?
After completing my postdoctoral research in a reputed institute, I would like to join academia in an Indian research institute, as a faculty, and guide the young minds of this country to the best of my capabilities.
Reference: Joshi A, Walimbe A, Avni A, Rai SK, Arora L, Sarkar S, & Mukhopadhyay S. Single-molecule FRET unmasks structural subpopulations and crucial molecular events during FUS low-complexity domain phase separation. Nat Commun. 14, 7331 (2023). https://www.nature.com/articles/s41467-023-43225-y
Edited by: Anjali Mahilkar
Biopatrika: Bringing Science to Society
© Biopatrika 2023 All Rights Reserved.

