Bacteria play an essential role in different ecosystems. Even though they are small, bacteria exhibit very complex dynamics in their lifestyle. They can self-organize into various collective phases in response to their surroundings. Among the numerous types and varieties of bacteria, self-propelled rod-shaped bacteria exhibit fascinating and diverse spatiotemporal phenomena. They use their flagella to move around, search for new resources and interact with each other and their environment. They can form dynamic clusters and move collectively, known as swarming.
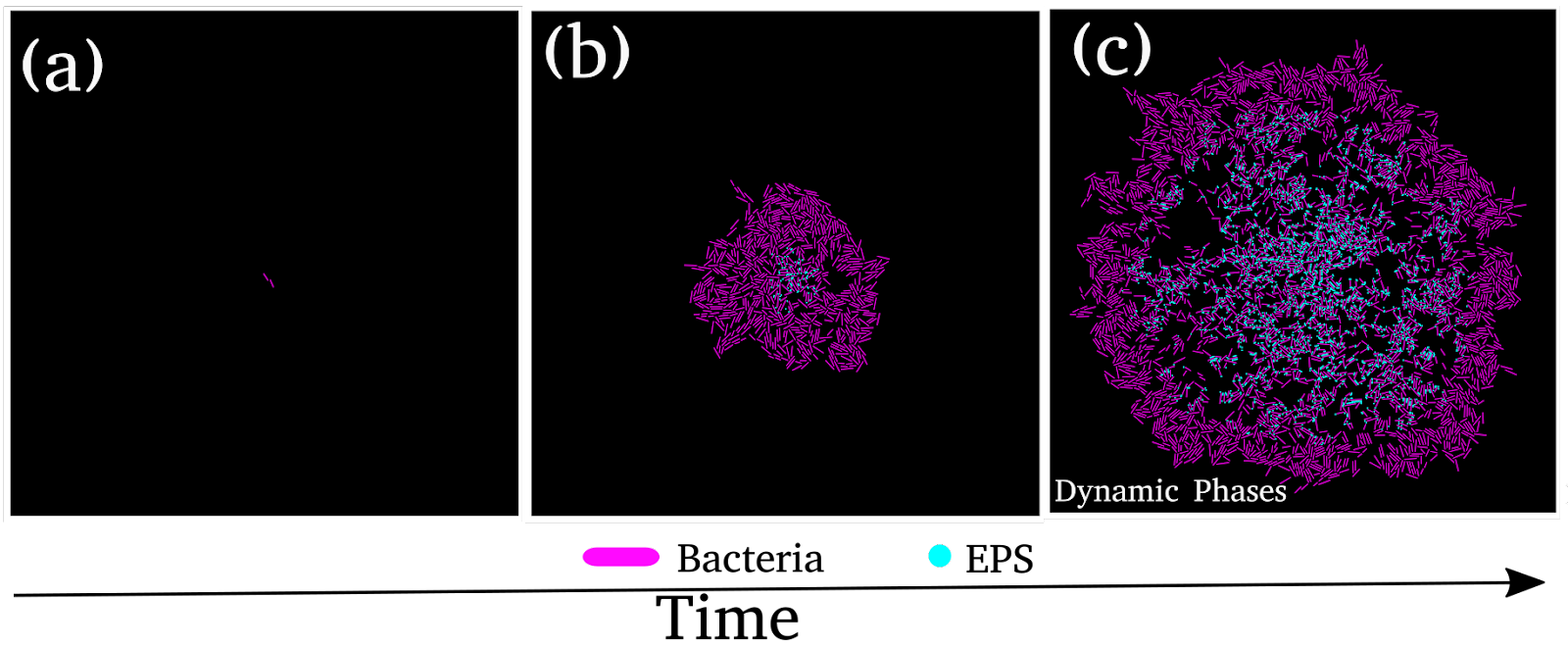
Depending on the environmental conditions, some species of bacteria can produce extracellular polymeric substances (EPS), essential in determining the structural and mechanical properties of the growing colony. EPS exhibits various properties, behaving like a sticky substance that binds individual bacterial cells together or acting as nonadsorbing to the surface of bacteria promoting repulsion between cells and EPS. In sticky EPS, bacteria form cellular aggregates trapped in a self-produced EPS.
Bacteria usually self-organize into a multicellular community known as a biofilm. Biophysical factors, including mechanical interactions, growth-induced stress, and motility-mediated dispersal, influence how motile bacteria transform into biofilm-like structures. However, the exact interplay among these factors is yet to be elusive.
Using computer simulations, we developed a particle-based biophysical model of rod-like bacteria in self-produced EPS. This simple biophysical model helps us to understand the spatiotemporal dynamics and different coexisting phases of a growing and expanding bacterial colony.
In brief, each rod-like motile bacterial cell can grow by consuming the local nutrient from the medium. Once the cell reaches a particular size, it will likely split into two daughter cells. Additionally, depending on the local nutrient availability, each cell can secrete EPS to its nearby region, which is sticky. This approach ensures that our simulated growing colony closely mimics an experimental setup. In our model, every agent interacts repulsively with one another, except for cells and EPS, which have an attractive interaction within a certain cutoff distance, reflecting the sticky properties of EPS. Moreover, they exhibit over-damped dynamics, indicating the medium’s highly viscous nature.
Using this model, we observed that when sticky EPS is present in the growth medium, the microcolony develops a coexistence of both motile and sessile aggregates, rendering the early stage of biofilm formation. The nutrient-dependent heterogeneous EPS production and the interplay between the growth and dispersion of the cells determine the fate of the multicellular growing colony. To conclude, our findings provide a significant understanding of biofilm morphogenesis and the coexistence of various phases.