About author
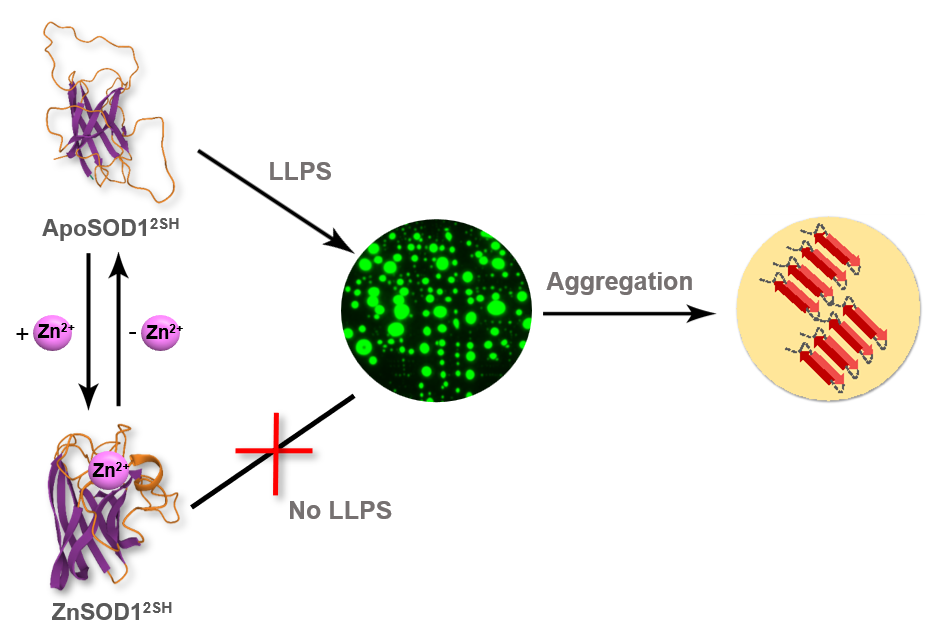
Bidisha Das pursued her Bachelor’s in Zoology from Calcutta University and Master’s in Life Sciences from Jawaharlal Nehru University, New Delhi. Bidisha qualified CSIR NET JRF in Life Science (2016). She joined Protein Folding Dynamics lab, CSIR-IICB for her doctoral studies in 2018 under the supervision of Dr. Krishnananda Chattopadhyay. Her research is interdisciplinary and involves delineating the role of metal ion cofactors in the aggregation of SOD1, that is associated with the motor neuron disease, ALS. She has received DBT Builder fellowship during her Master’s and the Carl Storm Diversity fellowship to attend 2022 Protein Folding Dynamics GRC held in Ventura, CA, USA.