Cells in our body can migrate very efficiently in the tissue, which requires continuous cell interactions with their underneath substratum (called extracellular matrix). Cell migration is controlled by a force-driven brake system called focal adhesion (FA), which is a multiprotein assembly of almost 200 proteins. Proper functioning of FA as a well-defined molecular clutch depends on its constituent mechanical linkages. These linkages both transmit and transduce the mechanical force into the cells. These FA proteins mechanically interact with each other and control the cell migration through traction force. Furthermore, these mutual interactions modulate the force-sensitivity of these proteins, which becomes dysregulated in different pathological conditions. Therefore, it is of utmost importance to decipher what plausible factors can modulate this force-response of these proteins.
Since each single protein molecule has their own contribution to the overall FA dynamics and thereby the cell migration, hence force-response of a single protein is of prime importance to understand the underlying mechanism. To date, many attempts have been performed to understand this mechanism, however, were unable to disclose what happens at single molecule level. Interestingly, we from the structural mechanobiology lab at Ashoka University have successfully addressed this problem and provided a plausible single-molecule scenario regarding the force sensitivity of these focal adhesion proteins.
Proteins are the fundamental units of cells and therefore, functional and structural maintenance of proteins is a first line mechanism for cells to survive. Molecular chaperones are such protein molecules which assists the cellular proteins to maintain their structure-function relation. These chaperones are also present in focal adhesion and emerging evidence suggest their contribution in focal adhesion dynamics. While existing studies have reported that chaperones assist in the folding of cytoplasmic proteins and prevent their aggregations, the researchers from the current study find that chaperones have a mechanical role too.
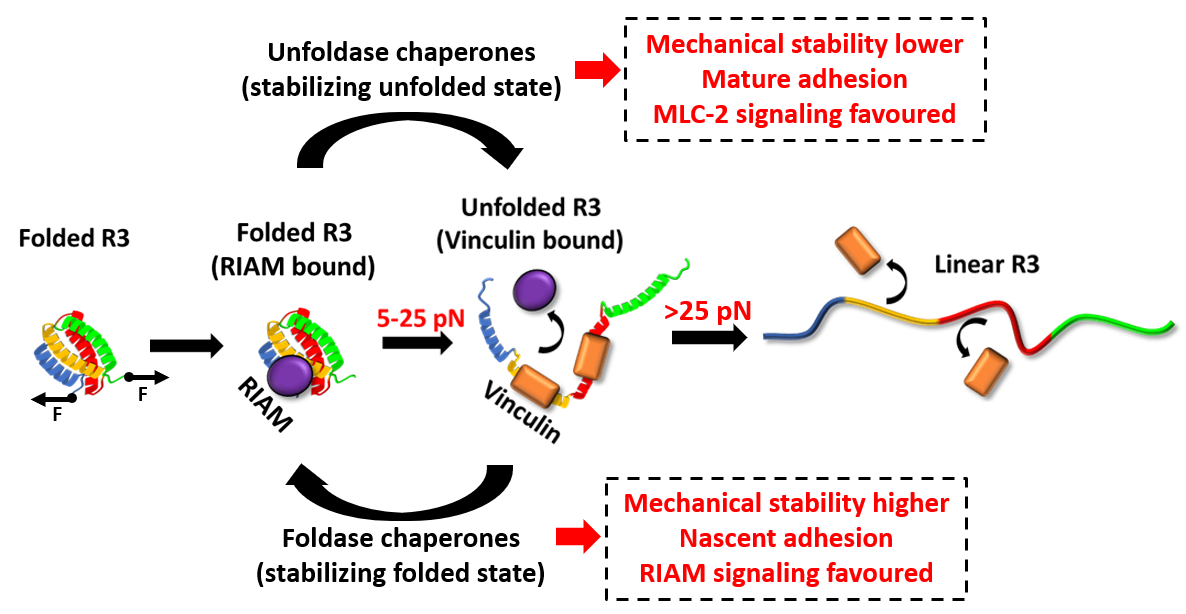
A single protein molecule has a force sensitivity within piconewton (pN) range and these chaperones are able to modulate the force sensitivity of these FA proteins. What does force sensitivity actually mean for a protein? In simple terms, force sensitivity is defined as the capability of proteins to withstand a certain amount of force while performing their biological functions. Since proteins have mainly two states: folded and unfolded. The energy is restored in the folded protein molecule, which could unfold depending on the force magnitude. Within their capacity, the protein molecules remain folded and accordingly interact with other proteins. However, at higher mechanical load beyond their ability, these folded proteins become unfolded since they cannot restore the energy anymore. This mechanical response dictates proteins to act as mechanical switches, controlling their interaction with other proteins and thereby, reflects into cellular behaviour.
Talin is a central FA protein, which exhibits this force-dependent folding behaviour and concurrent interactions. Within its 13 rod domains (R1-R13), R3 has least mechanical stability and unfolds very low force of 5 pN, whereas R13 domain has higher mechanical stability, unfolding at 15 pN. For example, at <5 pN force, talin interacts with Rap1 interacting adaptor molecule (RIAM), whereas at >5 pN force, talin unfolds and interacts with vinculin. This mechanical switching is mirrored in cellular structure and accelerates cell migration. Interestingly, the research group of the current study has observed that molecular chaperone can change this mechanical stability of talin.
We have developed the covalent magnetic tweezers (CMT), the first of its kind in India, to understand this force dependent-interaction of talin with chaperones. They found unfoldase chaperones (stabilizes the protein at mainly unfolded state) destabilize the folded talin and unfolds it at lower force. By contrast, the foldase chaperones increase the mechanical stability of talin. Consequently, this chaperone-modulated stability prompts changes in whole FA dynamics and cell behaviour.
Since chaperone overexpression is common in any pathological condition including cancer metastasis, where talin-centered FA dynamics play a central role; this force-dependent interaction signify a plausible mechanical effect of chaperone through inducing a domain-locked talin response in cell migration and consequently in its dysregulation.