Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.


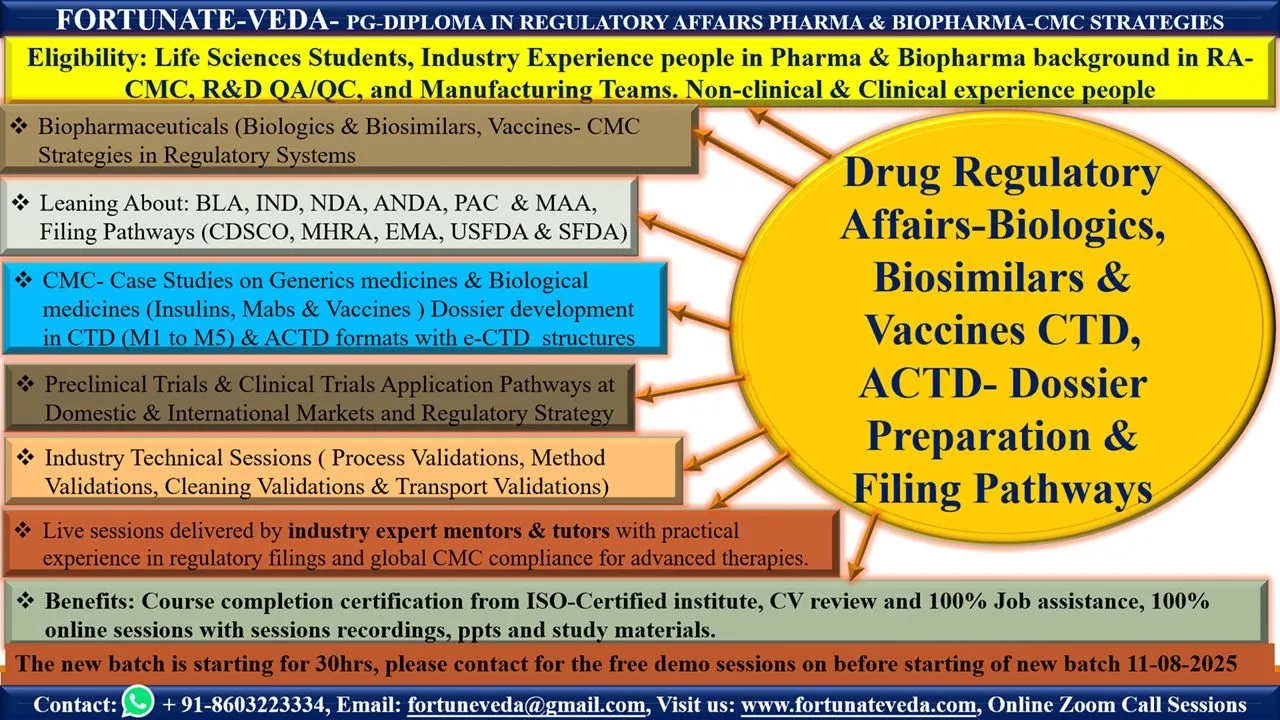



🎯 Course Title: Biologics, Biosimilars, and Vaccines CTD/ACTD Dossier Preparation & Filing Pathways
(Covering Regulated Markets, Rest of World (RoW), and India)
📅 Start Date: 11th August 2025 (Monday)
🕘 Timings: Monday to Friday | 9:00 PM – 10:30 PM IST
⏳ Duration: 30 Days | 30 Hours of Intensive Training
📍 Mode: Live Zoom Sessions
🔍 Course Highlights:
Comprehensive understanding of CTD and ACTD dossier structures
Region-specific regulatory filing strategies for Biologics, Biosimilars & Vaccines
Hands-on case studies & real-time examples
India, EU, US FDA, WHO, and other global filing insights
Ideal for Regulatory Affairs professionals, QA, QC, R&D, and Pharma/biotech graduates
📞 Contact Us:
🌐 Website: www.fortunateveda.com
📧 Email: fortuneveda@gmail.com
📱 Call/WhatsApp: +91-8603223334