📢 Online Course Announcement
🧬 Cell and Gene Therapy Regulatory CMC Strategies & Filing Pathway
Presented by: Fortunate-Veda Institute
🗓️ Course Start Date: 17th August 2025 (Sunday)
⏰ Time: 10:30 AM – 12:30 PM IST
📅 Schedule: Every Sunday | Weekly Sessions
Total Duration: 20 hours (Over 10 weeks)
💻 Mode: Online via Zoom
🎓 Session Coordinator & Tutor: Dr. Keerthana R
(Expert in Cell & Gene Therapy Regulatory Affairs)
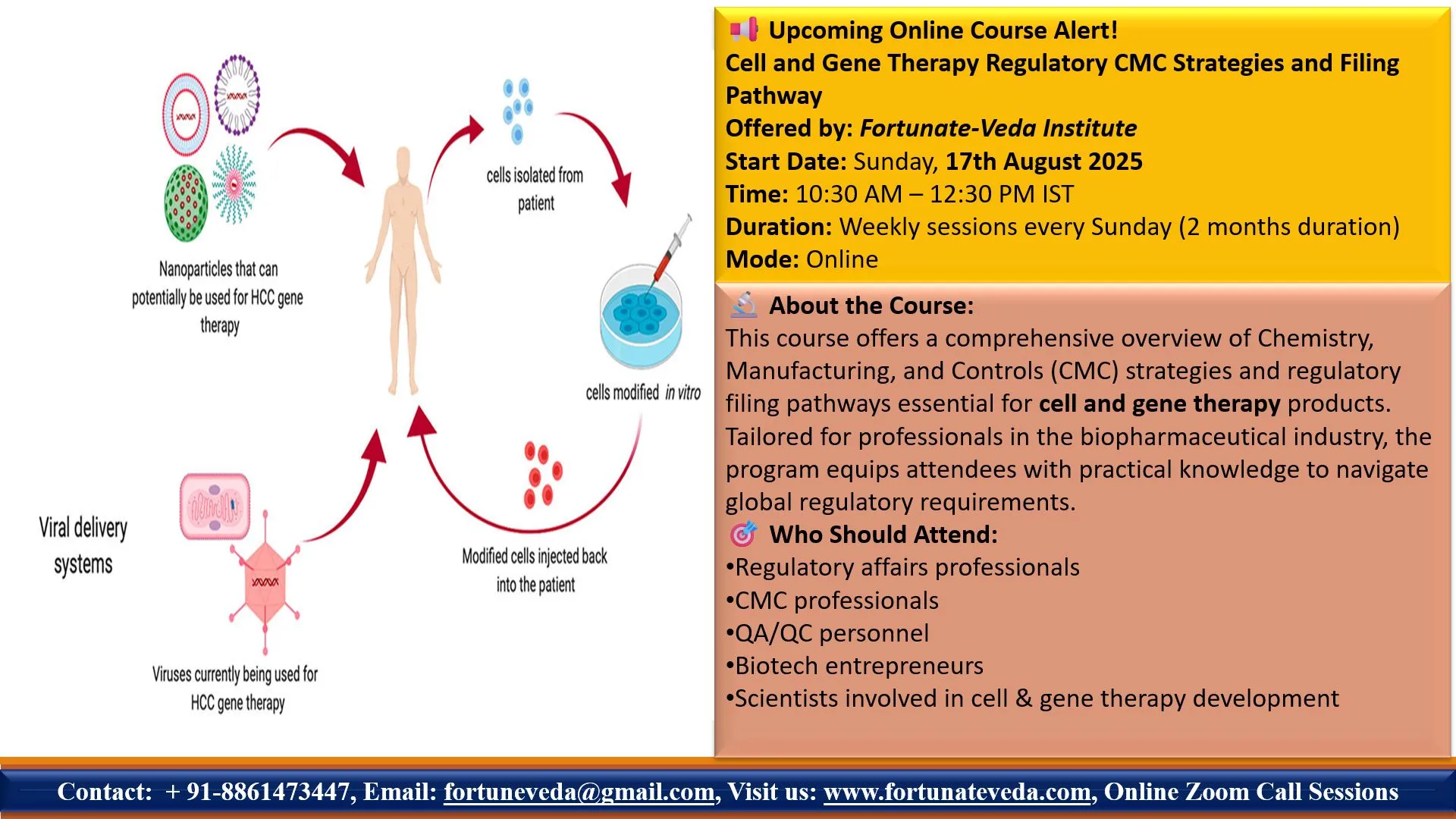
📘 Course Overview:
This expert-led online course will provide an in-depth understanding of regulatory CMC (Chemistry, Manufacturing, and Controls) strategies and filing pathways for cell and gene therapy (CGT) products. Ideal for biotech and pharmaceutical professionals aiming to stay ahead in this rapidly evolving field.
👥 Who Should Attend:
Regulatory Affairs Professionals
CMC, QA/QC, and Manufacturing Teams
Scientists and R&D Professionals
Pharma/Biotech Entrepreneurs
Students and Academicians in Life Sciences
✅ Key Highlights:
Deep dive into global CGT regulatory frameworks
IND, BLA, and other critical filing processes
CMC documentation strategy & compliance
Real-world case studies and regulatory insights
Interactive live sessions with Q&A
📞 Registration & Contact Info:
📱 Phone: +91-8861473447
📧 Email: fortuneveda@gmail.com
🌐 Website: www.fortunateveda.com
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




