📢 Online Training Program – Vaccines, Biologics & Biosimilars
Fortunate-Veda is organizing specialized online sessions on:
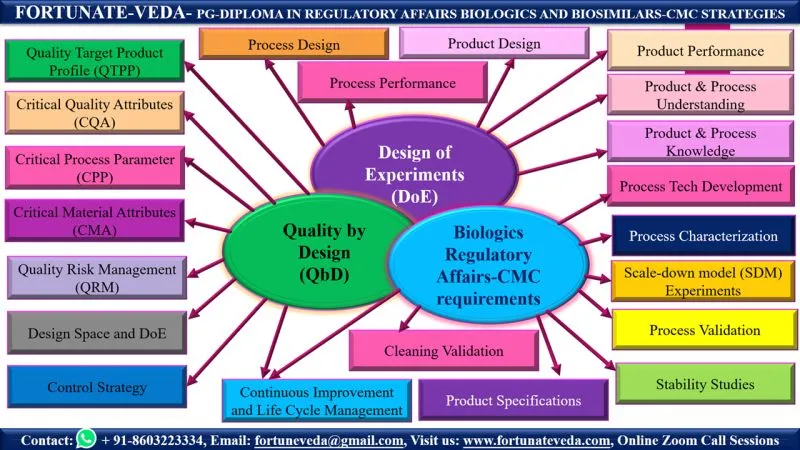
✅ Quality by Design (QbD)
✅ GMP Compliance
✅ Validations & Qualifications
✅ Data Integrity
✅ Regulatory Affairs – CMC Strategies
✅ Risk Assessment & Gap Assessment
✅ Scale-Down-Model (SDM) Experiments
✅ Process Characterization
✅ Technology Transfer
✅ Regulatory CMC Package Preparation
📅 New Chapter Starts: 06 October 2025
🕘 Schedule: Monday to Friday | 9:00 PM – 10:30 PM (IST)
📍 Live on Zoom
🎯 Gain practical, industry-focused knowledge to strengthen your expertise in CMC Development & Regulatory Affairs.
Register here: https://forms.gle/qUSZmjsWETjwcUCS6
📱 Queries to WhatsApp: +91-8603223334
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




