🎓 FORTUNATE-VEDA
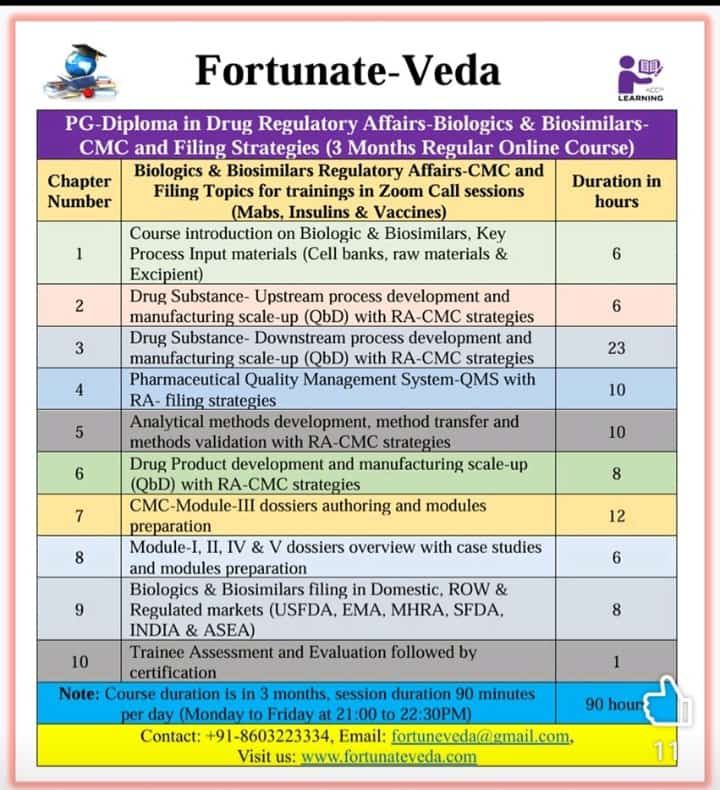
Postgraduate Diploma in Regulatory Affairs – Biologics & Biosimilars Vaccines Regulatory Filing Strategies
(CMC Strategies & Global Market Filing Pathways)
📢 New Batch | 3-Month Intensive Online Program (90 hrs)
🧬 Focus Areas:
Biologics, Biosimilars, Vaccines – Regulatory Affairs, CMC Strategies & Filing Pathways
🌍 Markets Covered:
CDSCO (India), USFDA (USA), EMA (Europe), Health Canada, PMDA (Japan), LATAM, TGA (Australia), SFDA (Saudi Arabia), ASEAN & more.
🗓️ Commencement Date: 15 September 2025
📆 Schedule: Monday to Friday
⏰ Timings: 9:00 PM – 10:30 PM (IST)
⏳ Duration: 3 Months (90 Hours)
📞 Contact Us:
WhatsApp: +91-8603223334
✉️ Email: fortuneveda@gmail.com
| contact@fortunateveda.com
🌐 Website: www.fortunateveda.com
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.