Research Summary: Spatial proximity imaging and proteomics characterize that splenic blood stem cells reside in a capsular niche, where their distribution is determined by theirs proliferative state under steady-state and stress conditions.
Author interview

Shubham Haribhau Mehatre is a PhD student in the Stem Cell Biology Laboratory at IISER Thiruvananthapuram, supervised by Dr. Satish Khurana. His doctoral research focuses on the maintenance and regulation of hematopoietic stem cells within distinct hematopoietic niches under steady-state and stress conditions.
Linkedin: https://www.linkedin.com/in/shubham-mehatre-0026a3196/
Twitter: @Shubham_Mehatre
Instagram: shubham_mehatre
Lab: Dr. Satish Khurana, Indian Institute of Science Education and Research Thiruvananthapuram
Lab social media/Twitter: @Satish__Khurana
Lab website: https://stemcelldevlab.wixsite.com/khuranalab
What was the core problem you aimed to solve with this research?
Apart from the BM, notable size of HSC pool is hosted by the spleen (Kiel & Morrison, 2008) among other tissues that are less significant in hematopoietic activity (Mende & Laurenti, 2021). While the role of splenic HSCs in daily blood cell production is not clearly evaluated, their equivalence with BM HSCs has been demonstrated (Morita et al, 2011). In fact, studies have shown that during development, splenic hematopoiesis is established by the incoming fetal liver and placenta-derived HSCs, even prior to the BM. Impairment in BM hematopoiesis, the ‘poised’ splenic HSCs engage in extramedullary hematopoiesis (EMH). Physiological states such as pregnancy (Nakada et al, 2014) and aging (Nilsson & Bertoncello, 1994); pathological states such as anemia (Bennett et al, 1968), myeloablation (Morrison et al, 1997), blood loss (Cheshier et al, 2007), and several types of infections (Baldridge et al, 2010) display an active engagement of splenic hematopoiesis. What role do the spleen resident HSCs play during EMH is not well understood. Interestingly, the functional robustness of splenic HSCs with a probable role in maintaining hematopoietic homeostasis was demonstrated (Wolber et al, 2002). However, little is known about the molecular regulation of splenic HSC function.
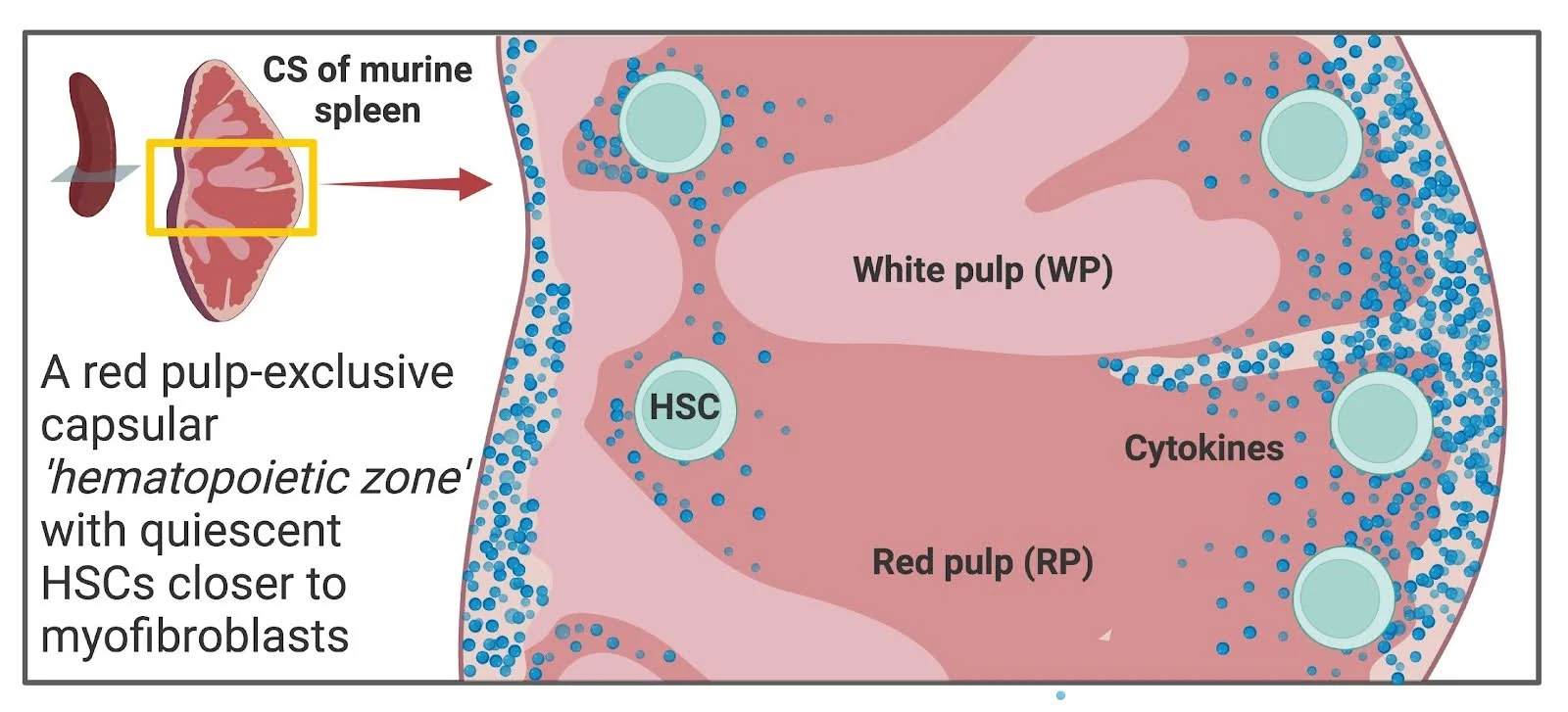
How did you go about solving this problem?
To localize primitive hematopoietic stem cells (pHSCs) in the spleen, we used immunofluorescence staining based on the established Lin⁻ CD41⁻ c-Kit⁺ Sca-1⁺ CD150⁺ CD48⁻ phenotype. Spatial mapping in spleen sections revealed that pHSCs reside predominantly in a subcapsular “hematopoietic zone” in close proximity to αSMA⁺ myofibroblasts. Quantitative spatial analysis confirmed their preferential localization near this fibrous capsular layer. We assessed sex-specific differences and found that pHSCs in female mice were significantly closer to the capsule compared to males. Given the vascular niche’s importance in bone marrow (BM), we evaluated blood (CD31⁺) and lymphatic (Lyve-1⁺) vessels in the spleen but found these structures largely excluded from the red pulp, suggesting a vascular-independent niche for splenic pHSCs. To examine the effect of HSC activation on niche association, we administered G-CSF and analyzed pHSC positioning at peak EMH and one month post-treatment. G-CSF caused a transient displacement of pHSCs away from the capsule, which reversed upon return to quiescence. A similar shift was observed following 5-FU–induced myeloablation, further linking proliferation with loss of niche proximity. Finally, quantitative proteomic analysis of sorted myofibroblasts versus stromal cells confirmed a unique expression profile enriched for ECM-related and HSC-regulatory proteins in myofibroblasts. Together, our approach integrates spatial imaging, functional perturbations, and proteomics to define a novel capsular myofibroblastic niche that maintains quiescent pHSCs in the spleen.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Our research discovered that a special group of blood-forming stem cells (called pHSCs) in the spleen live in a unique area just beneath the organ’s outer capsule, where they are closely associated with supportive cells called myofibroblasts. Unlike in the bone marrow, where blood vessels play a major role in supporting stem cells, in the spleen these myofibroblasts form a distinct “niche” that helps stem cells stay healthy and in a resting (quiescent) state. We found that this location is sensitive to changes: when the stem cells are activated—either by drugs like G-CSF or 5-FU—they move away from the capsule, but return once they return to a resting state, showing that their position is closely linked to their activity. Interestingly, female mice had stem cells positioned closer to the capsule than males, suggesting sex-based differences in how this niche functions. We also found that the myofibroblasts produce key molecular signals that support stem cells, and their interaction with stem cells is stronger than with other spleen cells. Overall, this is the first evidence of a specialized myofibroblast-based niche in the spleen that supports blood stem cells during steady and stress conditions, offering new insights into how blood formation is maintained outside the bone marrow.
What are the potential implications of your findings for the field and society?
Our findings identify a previously unrecognized myofibroblast-rich capsular niche that supports quiescent hematopoietic stem cells (HSCs) in the spleen under steady-state conditions. This discovery has several important implications. First, it advances our understanding of stem cell biology by providing a simpler and more defined niche model compared to the heterogeneous bone marrow environment, enabling deeper investigation of niche-derived regulatory cues. Second, the molecular features of this splenic niche may offer novel targets to modulate HSC behavior, with potential applications in enhancing ex vivo expansion or improving engraftment efficiency in transplantation settings. Third, our findings have direct clinical relevance, as HSCs often become sequestered in the spleen during bone marrow transplantation and donor mobilization; understanding the mechanisms behind this retention could inform strategies to prevent stem cell loss and improve therapeutic outcomes. Finally, this work provides insight into the spleen’s role in stress-induced extramedullary hematopoiesis (EMH), which may be relevant to managing hematologic disorders characterized by aberrant EMH, such as myelofibrosis or chronic inflammation. Collectively, our study redefines the spleen as a physiologically significant site of HSC regulation with translational potential in regenerative medicine and hematologic disease.
“This study reveals how splenic capsular niches spatially organize and maintain HSCs, revealing proliferative state-dependent positioning during homeostasis and stress.”
What was the exciting moment during your research?
One of the most exciting moments during our research was observing the consistent and spatially restricted localization of primitive HSCs near the splenic capsule, tightly associated with myofibroblasts. This unexpected and well-defined pattern stood out immediately under the microscope and hinted at a previously unrecognized, organized niche structure in the spleen. It was particularly striking because such spatial clarity is rare in hematopoietic tissue, especially compared to the complex and diffuse bone marrow environment. This observation was the turning point that led us to systematically investigate the capsular region and ultimately define a novel splenic HSC niche.
Paper reference: Mehatre SH, Sanam S, Agrawal H, Hejjaji AV, Kumar AS, Alam MT, Khurana S (2025) A capsular myofibroblastic niche maintains hematopoietic stem cells in the spleen. The EMBO Journal: 1-30-30. https://www.embopress.org/doi/full/10.1038/s44318-025-00477-2
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




