Research Summary: The story of complex life is, at its core, the story of the cytoskeleton—the internal scaffolding that gives cells their shape, strength, and ability to divide. While we know how crucial this machinery is in modern eukaryotic cells, its earliest evolutionary steps remain elusive. Since their discovery in 2015, Asgard archaea have drawn attention as the closest known relatives of eukaryotes, carrying many eukaryotic signature proteins (ESPs) once thought unique to complex cells. Yet, the way they divide and organize their interiors is still a mystery.
In our study, we focused on Odinarchaeota yellowstonii, an Asgard archaeon isolated from Yellowstone National Park, to probe this transition. We examined two of its FtsZ proteins—ancient relatives of tubulin, the building blocks of eukaryotic microtubules. Using phylogenetic analysis, biochemical assays, and cryo-electron microscopy, we discovered that these related proteins behave in remarkably different ways: one assembles into straight protofilaments, like strings, while the other coils into spiral structures, like springs. Even their strategies for attaching to the cell membrane diverge; one connects directly, while the other requires a helper protein.
This striking “division of labor” within the same cell offers a glimpse of how functional specialization may have begun billions of years ago. By showing how Asgard proteins diversified their roles, our findings bridge a critical evolutionary gap between the simple filaments of microbes and the intricate cytoskeletal systems that allow modern cells including our own to grow, move, and divide.
Author Interview

Jayanti Kumari is a PhD student at the Department of Biochemistry, Indian Institute of Science (IISc). After completing her B.Tech in Biotechnology, she began her doctoral research on archaeal cytoskeletons and cell division. Her work focuses on Asgard archaea recognized as the closest living relatives of eukaryotes and examines how their cytoskeletal proteins evolved to bridge the gap between simple prokaryotic filaments and the complex architectures of modern eukaryotic cells.
Twwitter: https://x.com/Jayantikri23
Lab: Dr. Saravanan Palani, Synthetic cell Biology Lab, Department of Biochemistry, Indian Institute of Science, Bangalore
Lab website: https://syncellbiolab.weebly.com
What was the core problem you aimed to solve with this research?
Although every modern eukaryotic cell depends on a sophisticated cytoskeleton, the evolutionary steps that transformed simple microbial filaments into these complex networks remain poorly understood. While bacteria and many archaea rely on FtsZ for division, eukaryotes replaced it with actin- and tubulin-based systems, enabling advanced functions such as chromosome segregation.
Asgard archaea are particularly fascinating in this context because they are the closest known relatives of eukaryotes and carry many eukaryotic signature proteins (ESPs) once believed to exist only in complex cells. Remarkably, some Asgard lineages retain both FtsZ and tubulin-like proteins, raising a critical evolutionary question: how did these two systems coexist within the same cell, and what drove their eventual specialization?
Odinarchaeota, with its unusual combination of cytoskeletal proteins, provides a rare living window onto this transition. By investigating its FtsZ paralogs, we aimed to uncover how early cytoskeletal systems diversified, shedding light on one of the key evolutionary steps that shaped the internal architecture of modern eukaryotes.
How did you go about solving this problem?
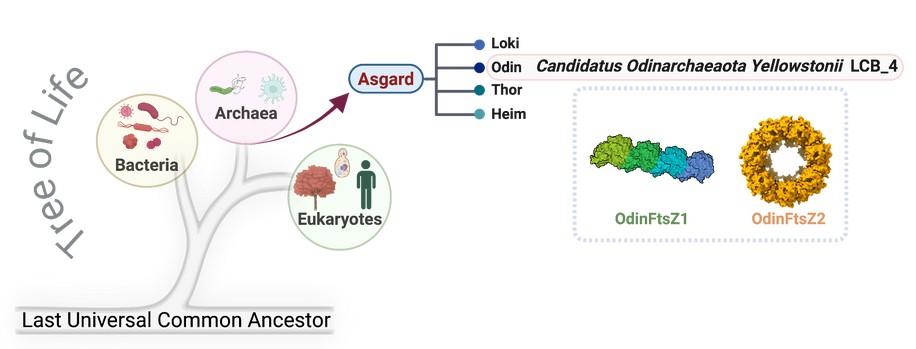
In our research, we focused on Odinarchaeota yellowstonii, named after the Norse god Odin and first isolated from Yellowstone National Park, USA. This lineage is fascinating because it encodes multiple FtsZ proteins, part of the tubulin superfamily that later gave rise to the building blocks of eukaryotic microtubules.
We investigated two of these proteins, OdinFtsZ1 and OdinFtsZ2, using phylogenetic analysis, biochemical assays, and cryo-electron microscopy. Evolutionary analysis confirmed that they belong to two distinct archaeal FtsZ subfamilies. Functionally, our experiments revealed that OdinFtsZ1 assembles into straight protofilaments, like strings, while OdinFtsZ2 organizes into stacked spiral rings, resembling tightly coiled springs.
We also observed key differences in membrane attachment: OdinFtsZ1 binds directly via an amphipathic helix, whereas OdinFtsZ2 relies on a helper anchor protein, OdinSepF. Together, these findings demonstrate that even closely related proteins can employ distinct mechanisms-evidence of functional specialization already underway in ancient archaeal cells.
How would you explain your findings to the non-scientific community?
Every living cell has an internal “skeleton” that gives it shape, helps it move, and allows it to divide, much like the frame of a building or the bones in our bodies. In our research, we studied two ancient proteins that can be thought of as early building blocks of this cellular skeleton. Although they are related, we found they behave in dramatically different ways. One builds straight filaments, like laying down strings in a line, while the other twists into spiral coils, like springs. They also attach to the cell surface in different ways: one binds directly, while the other needs a helper protein.
This early form of teamwork and specialization may have been one of the first evolutionary steps toward the complex skeletons that modern cells use to grow, move, and organize themselves. In other words, these tiny proteins in ancient microbes may represent a glimpse of how life’s inner scaffolding first began to evolve.
Scientific Insights in Detail
- Distinct filament morphologies: OdinFtsZ1 resembles bacterial FtsZ filaments, while OdinFtsZ2 forms stacked spirals similar to OdinTubulin or FtsA. This indicates potential cooperative or specialized roles in cell division.
- Membrane interactions: OdinFtsZ1 directly binds membranes, while OdinFtsZ2 depends on SepF. Similar patterns are seen in other archaea with two FtsZ paralogs.
- Evolutionary significance: The co-existence of FtsZ and tubulin-like proteins in Asgard archaea may represent a transitional stage toward multi-protofilament tubules in eukaryotes.
- Genomic context: OdinFtsZ genes are located near ribosomal and tRNA synthetase genes, suggesting ancient links between protein synthesis and cell division regulation.
What are the potential implications of your findings for the field and society?
One of biology’s grand questions is how simple microbial cells gave rise to the complex architecture of eukaryotic life. Since their discovery in 2015, Asgard archaea have been recognized as the closest living relatives of eukaryotic cells, yet how they divide and organize internally has remained a mystery. By showing that their FtsZ proteins can adopt dramatically different filament shapes and membrane-attachment strategies, our study reveals that cytoskeletal specialization and division of labor emerged much earlier in evolution than previously thought.
For the field of cytoskeleton and cell biology, these findings position Asgard archaea as living “intermediates,” uniquely bridging prokaryotic simplicity with eukaryotic complexity. For society, they enrich our understanding of life’s deep history, showing that the cellular systems enabling growth, healing, and reproduction in all organisms, including humans, were built on innovations first tested billions of years ago. In essence, by examining these microbes, we retrace some of the earliest evolutionary steps that made complex cellular life, and ultimately our own existence, possible.
“Our study reveals how ancient proteins first began to specialize, laying the groundwork for the sophisticated cytoskeletons that define modern cellular life. By tracing distinct filament architectures in Asgard archaea, we uncover how evolution may have shaped eukaryotic cells and exposed the remarkable diversity of cytoskeletal systems in early microbes. These findings reinforce the view that the eukaryotic cytoskeleton has deep archaeal roots, as Asgard archaea encode tubulin, actin homologs, and primitive FtsZ-like proteins offering a living bridge between simple prokaryotic systems and the complexity of eukaryotic cell biology. — Dr. Saravanan Palani”
What was the most exciting moment during your research?
The true “Eureka moment” came when we realized that a single archaeal cell, Odinarchaeota LCB_4, carries not one but three distinct tubulin-family proteins FtsZ1, FtsZ2, and OdinTubulin. Such coexistence is extremely rare and has only been reported in a handful of Asgard lineages.
The excitement grew when we looked at them under cryo-electron microscopy. Before our eyes, the proteins revealed their personalities: OdinFtsZ1 assembled into the neat, classical protofilaments of bacteria, while OdinFtsZ2 formed striking stacked spirals that closely resembled OdinTubulin. Seeing these distinct architectures within the same cell was like watching evolution frozen in action an ancient microbial cytoskeleton experimenting with diversity, bridging the gap between simple bacterial filaments and the complex microtubules of modern eukaryotes.
Reference: Kumari J, Uthaman A, Bose S, Kundu A, Sharma V, Dutta S, Dhar A, Roy S, Srinivasan R, Pande S, Vinothkumar KR, Gayathri P, Palani S. Distinct filament morphology and membrane tethering features of the dual FtsZ paralogs in Odinarchaeota. The EMBO Journal (2025). https://doi.org/10.1038/s44318-025-00529-7
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




