Origin of structural heterogeneity in amyloid fibrils associated with disease AL amyloidosis
Research Summary: Here, we have characterized the early aggregation events, including structural and biophysical features of aggregation competent monomers and how this monomer, upon providing adequate conditions, turns into highly stable amyloid assemblies.
Author interview

Sharvari Palkar, Ishaan Chaudhary and Basudha Patel were my first set of trainees in the lab who contributed to this work along with me. All three of them are truly exceptional. They accomplished this significant work while the lab was still being established. Joining on 9th September 2024 and achieving a published paper by October 2025 shows that both lab setup and research progressed hand in hand, supported by my collaborators, Dr. Amit Kumawat and Dr. Shang-Te Danny Hsu. This was possible only because of these hardworking and highly motivated students, who were willing to take on every challenge that came their way
Linkedin: https://www.linkedin.com/in/saritapuri
Twitter: @Saritapuri24
Lab: Sarita Puri, Indian Institute of Science Education and Research Pune, India
What was the core problem you aimed to solve with this research? Through this research, we aimed to establish the origin of amyloid polymorphism in systemic amyloid disease, AL amyloidosis.
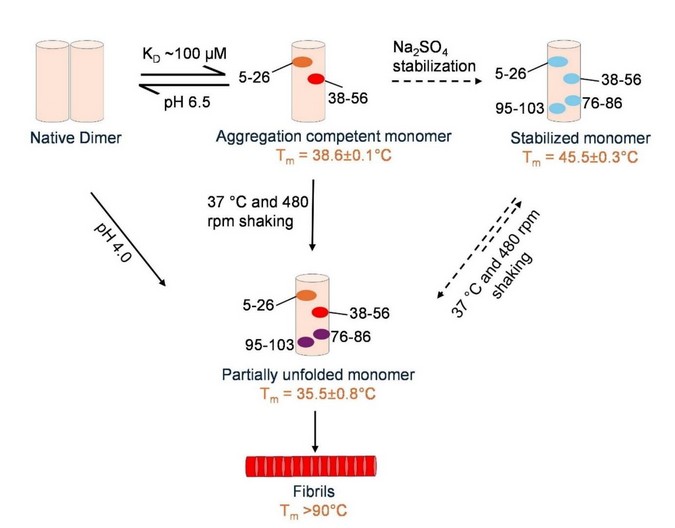
How did you go about solving this problem: We applied a biophysical and structural biology approach to investigate the aggregation pathway of an isolated variable domain of light chain derived from an AL patient.
How would you explain your research outcomes (Key findings) to the non-scientific community: In AL amyloidosis, harmful protein fibers of antibody light chains can form many different shapes, but we didn’t know why. Our research shows that this variation starts very early: even single protein units can take slightly different shapes before they clump together. These early differences lead to different types of amyloid fibers. In short, small changes at the beginning determine the kind of harmful fibers that finally form.
My lab’s first publication within a year of joining at IISER Pune. It also received appreciative feedback from the handling editor, which makes this recognition truly special. — Dr. Sarita Puri
What are the potential implications of your findings for the field and society: The detailed structural characterization of early aggregation events is useful to target them with protein stabilizers, which can prevent aggregation. Ultimately, this strategy will be useful in deposition of harmful fibrils in vital organs like the heart, kidneys, and other organs, which is the cause of death in AL amyloidosis patients.
What was the exciting moment during your research: The Calculation of the dimer dissociation constant and its correlation with concentration-dependent aggregation kinetics was an interesting and exciting moment to carry this research further.
Paper reference: Puri, S.*, Palkar, S., Kumawat, A., Chaudhary, I., Patel, B., Kumar, V. V., … Ricagno, S. (2025). Dimer Dissociation and Aggregation Hot-spot Exposure Synergistically Accelerate Light Chain Variable Domain Aggregation Associated With AL Amyloidosis. Journal of Molecular Biology, 437(24), 169468. https://doi.org/10.1016/j.jmb.2025.169468
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




