Membrane lipid environment modulates function and bioenergetic control in human mitochondrial VDAC3
Research Summary: We show that the lipid microenvironment, rather than protein sequence alone, dictates human VDAC3 stability and function. Cardiolipin selectively disrupts human VDAC3 voltage-dependent gating, potentially linking mitochondrial lipid remodeling to apoptosis.
Researcher spotlight

Aadish Rawat is a Ph.D. candidate in Prof. Dr. Radhakrishnan Mahalakshmi’s Molecular Biophysics Laboratory at Indian Institute of Science Education and Research, Bhopal.
Linkedin https://in.linkedin.com/in/aadish-rawat
ResearchGate https://www.researchgate.net/profile/Aadish-Rawat
Twitter https://x.com/aadish_rawat
Lab: Prof. Dr. Radhakrishnan Mahalakshmi, Indian Institute of Science Education and Research, Bhopal
Lab website: https://home.iiserb.ac.in/~maha/
What was the core problem you aimed to solve with this research?
Interactions between transmembrane proteins and their lipid environment are critical for protein stability and function, yet the molecular factors governing this regulation remain poorly defined. Using human VDAC3, the evolutionary parent of human VDAC isoforms, as a model mitochondrial β-barrel, we aimed to determine whether membrane physicochemical and mechanical properties actively regulate VDAC behavior or merely provide a permissive scaffold for folding and function. We further asked which outer mitochondrial membrane lipids shape VDAC conformational dynamics, stability, and voltage-gated function.
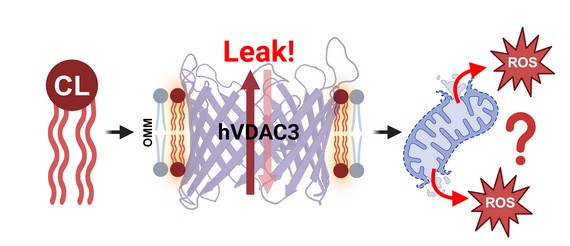
How did you go about solving this problem?
We designed 19 systematically varied lipid environments spanning physiological, simplified, and stress-mimetic membranes, and integrated electrophysiology, equilibrium energetics, and all-atom simulations to determine how specific lipid properties influence human VDAC3 behavior. This approach allowed us to compare channel behavior across well-controlled membrane contexts.
“We report the first discovery of how hVDAC3-membrane interaction and cardiolipin-mediated regulation together dictate mitochondrial health and oxidative stress adaptation.” – Prof. Dr. Radhakrishnan Mahalakshmi
How would you explain your research outcomes (Key findings) to the non-scientific community?
We discovered that the lipids surrounding VDACs don’t just hold them in place; they actively influence how these channels function. Some lipids support normal channel activity, while others, such as cardiolipin, can lock the channel in an open-like, high-conductance state, which may alter mitochondrial permeability and contribute to cellular stress and programmed cell death.
What are the potential implications of your findings for the field and society?
Our findings highlight membrane lipids as active regulators of mitochondrial function, rather than mere passive structural components. By showing how lipids can directly modulate human VDAC3 behavior, this work provides a framework for interpreting VDAC function, predicting mitochondrial responses under non-physiological conditions, and studying context-dependent channel behavior. Beyond advancing fundamental mitochondrial biology, these insights can aid the modulation of mitochondrial function during lipid dysregulation, in which VDACs play a central role.
What was the exciting moment during your research?
An exciting moment during our research was discovering that the previously-reported poor gating of human VDAC3 in simplified membranes was effectively mitigated in several physiological lipids. Watching its function recover and respond sensitively to membrane composition revealed just how powerfully the lipid microenvironment shapes channel behavior.
Paper reference: Rawat A, Mahalakshmi R. Protein–lipid interplay governs ion channel gating and bioenergetics in human mitochondrial VDAC3. Protein Science. 2026; 35(2):e70486. https://doi.org/10.1002/pro.70486
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




