Research Summary: We show that β-CATENIN, a key player in Wnt signaling pathway plays a crucial role in positioning the midline guidance cells, which helps in corpus callosum crossing.
Author interview

Dr. Arpan Parichha, currently a scientist at CSIR-IGIB, completed his PhD at TIFR, Mumbai, under Prof. Shubha Tole. His research explores molecular and cellular mechanisms of neurodevelopment and their disruption in disorders like autism.
Linkedin: https://www.linkedin.com/in/arpan-parichha-390859167/
Twitter: https://x.com/arpan_parichha
Instagram:https://www.instagram.com/arpanparichha/
Lab: Prof Shubha Tole, Tata Institute of Fundamental Research, Mumbai
Twitter: https://x.com/shubhatole
What was the core problem you aimed to solve with this research?
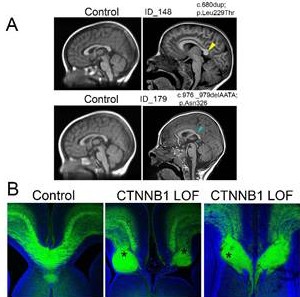
Mutations in the CTNNB1 gene, which encodes β-CATENIN, are associated with defects in the corpus callosum—the bridge that links the two halves of the brain. The cellular basis of this phenotype has remained unclear. In our work, we investigated how loss or gain of β-CATENIN affects specific cell types involved in corpus callosum development. We found that β-CATENIN’s function in midline guidance cells is essential for proper corpus callosum formation.
How did you go about solving this problem?
We analyzed transgenic mouse lines with β-CATENIN gain-of-function (GOF) or loss-of-function (LOF) targeted specifically to midline guidance cells using an Lmx1aCre driver. Our fate mapping experiments show that the Lmx1a lineage gives rise to midline cell types essential for corpus callosum guidance, including the glial wedge, indusium griseum glia, and a subset of glutamatergic neurons. Both β-CATENIN GOF and LOF embryos exhibited abnormalities in these structures, leading to severe disruption of corpus callosum crossing and the formation of Probst bundles. These results demonstrate that precise regulation of β-CATENIN activity in midline guidance populations is crucial for corpus callosum formation and is possibly mis-regulated in CTNNB1 syndrome, where human patients carry mutations in the β-CATENIN gene.
“This was an unexpected finding – we didn’t expect such a dramatic disruption of the corpus callosum from Lmx1a lineage-specific gain or loss of Ctnnb1. It’s to Arpan’s credit that he followed it up tenaciously and took the initiative to set up a collaboration that revealed an evolutionary conservation of CTNNB1 function in this major brain connectivity pathway. Delighted that we can now showcase this exciting finding via Biopatrika as well.” – Prof. Shubha Tole
How would you explain your research outcomes (Key findings) to the non-scientific community?
The right and left halves of our brain are connected by a gigantic bridge of nerve fibers called the corpus callosum. In many neurodevelopmental disorders, including the rare genetic condition CTNNB1 syndrome, this bridge does not form properly, leading to defects in brain function. We wanted to understand in which cell type the function of β-CATENIN (encoded by the CTNNB1 gene) is most critical for this defect to occur. Our research revealed that a group of cells present in the embryonic brain, known as midline guidance cells, act like traffic lights, helping nerve fibers navigate from one side of the brain to the other. When β-CATENIN function is either lost or gained, these cells become disorganized, leading to failed axon navigation. As a result, misrouted axons pile up, forming what is called a Probst bundle. This is similar to a traffic jam, if traffic signals are missing or misplaced, vehicles get diverted resulting in chaos.Taken together, our findings provide a cellular-level insight into how CTNNB1 mutations disrupt brain wiring, helping to gain deeper insight to the underlying causes of this disease.
What are the potential implications of your findings for the field and society?
CTNNB1 syndrome is a rare genetic disorder, and the pathophysiology underlying most of its clinical symptoms remains poorly understood. One such clinical observation is the defect in the corpus callosum, for which the underlying cause or mechanism was previously unknown. In this context, our findings provide new cellular-level insights into the corpus callosum defects observed in CTNNB1 syndrome. Since CTNNB1, which encodes β-catenin, is ubiquitously expressed in the brain, identifying the specific cell types in which its function is critical for corpus callosum development offers researchers a more targeted framework for future cell and gene therapies.
This research is particularly special to me, as it was one of three discoveries we initially made with the CTNNB1 gain-of-function mice. This project began in 2021, a collaboration between me and a fellow student Varun Suresh. It took us several years to bring this work to fruition. One major step was when I connected with the CTNNB1 Foundation and establish a global collaboration with Dr. Damjan Osredkar, Department of Pediatric Neurology, University Children’s Hospital, Ljubljana, Slovenia, and Dr. Špela Miroševič, Co-Founder of the CTNNB1 Foundation in Ljubljana, Slovenia, and mother of a son diagnosed with CTNNB1 syndrome.The guidance and support of my mentor, Prof. Shubha Tole gave me the confidence to pursue an independent career in academia. After I started my own laboratory at CSIR-IGIB, I could involve my student Shreya Yaday in a part of the revision process, which was a particularly exciting milestone for me. Importantly, this was my first study aimed at uncovering the fundamental biology of a clinically less understood rare syndrome, with the goal of providing new insights and contributing to the global scientific community.
Reference: Parichha, A., Datta, D., Singh, A., …, Gosar, D., Osredkar, D., & Tole, S. (2025). An evolutionarily conserved role for CTNNB1/β-CATENIN in regulating the development of the corpus callosum. iScience, 28(9), 113335. https://www.cell.com/iscience/fulltext/S2589-0042(25)01596-2
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




