Research Summary: We discovered that biomolecular condensates can reverse the enantioselectivity of alkaline phosphatase, offering insights into enzyme adaptability and potential applications in chiral synthesis and protocell studies.
Author interview

Shikha is from New Delhi. She had completed her Bachelor’s in Chemistry from Shaheed Rajguru College of Applied Sciences, Delhi University in 2019 and joined as an Integrated Ph.D. student at IISER Mohali and currently pursuing Ph.D. on biomolecular chemistry at IISER Mohali under the supervision of Dr. Subhabrata Maiti. She is currently focusing on enzyme behavior in synthetic cellular environments and the role of chirality in early life processes.
Linkedin: www.linkedin.com/in/shikha-sharma-21a21822b
Twitter: https://x.com/Shikha3342
Lab: Dr. Subhabrata Maiti, Indian Institute of Science Education and Research (IISER), Mohali
Twitter: https://x.com/Subhachem
What was the core problem you aimed to solve with this research?
The core problem this research aimed to solve was understanding how the enantioselectivity of enzymes, particularly alkaline phosphatase (ALP), is affected by different biomolecular condensate environments. Traditionally, enzymes like ALP exhibit a fixed preference for one enantiomer over the other—in this case, a preference for the S-enantiomer of 2-hydroxypropyl-p-nitrophenyl phosphate (S-HPNPP). However, the study hypothesized that this enantioselective behavior might be altered when the enzyme is encapsulated within biocondensates, such as protein- or DNA-based coacervates. This question is of significant relevance because biocondensates create a crowded and dynamic internal environment, which may change enzyme conformations, substrate binding affinity, and reaction dynamics. Prior studies have shown how condensates influence overall enzymatic activity, but not how they affect selectivity toward enantiomers. This research directly addressed that gap, revealing that enzyme selectivity can not only be tuned but completely inverted depending on the internal environment of the condensate, thus challenging the conventional understanding of enzymatic specificity and opening avenues in synthetic biology and chiral catalysis.
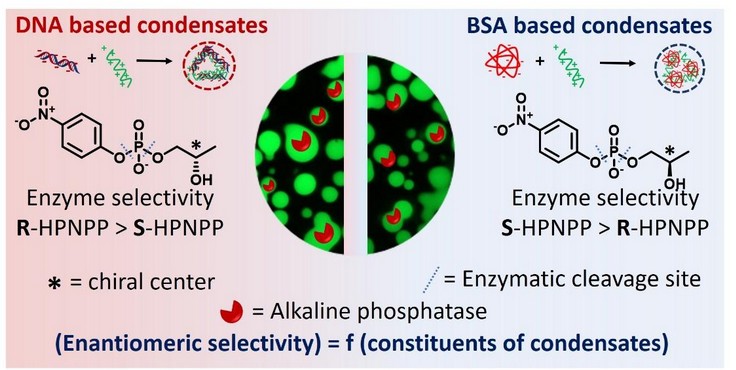
How did you go about solving this problem?
We synthesized chiral RNA-like substrates and we measured ALP’s activity toward both R- and S-HPNPP enantiomers using kinetic assays to compare enantioselective behavior, confocal imaging, FRAP, REES, fluorescence microscopy, and spectroscopy to assess enzyme behavior, mobility, and conformational changes within each condensate environment.
“Inside biomolecular condensates, enzyme function is not just preserved—it is redefined. — Dr. Subhabrata Maiti”
How would you explain your research outcomes (Key findings) to the non-scientific community?
Imagine enzymes as tiny machines in your body that perform specific tasks, like breaking down food or building important molecules. Just like how a key fits a certain lock, these machines often prefer working with one version (or “hand”) of a molecule—either left-handed or right-handed.
In our research, we discovered something surprising: when we placed one such enzyme into a special kind of environment called a biomolecular condensate (think of it like a soft, jelly-like compartment inside cells), its preference for the “handedness” of a molecule completely changed.
In normal water-like conditions, our enzyme (called ALP) liked the “S” version of a test molecule. But when we put it into a DNA-based condensate, it suddenly preferred the “R” version instead. It was as if the enzyme had flipped its personality depending on its surroundings!
Why is this important? Because it shows that enzymes are more flexible than we thought—they can change their behavior based on where they are. This insight helps us better understand how early life might have started, how cells work without membranes, and how we might design new ways to make useful chemicals, especially in medicine.
In simple terms: we showed that enzymes can change their behavior just by changing their environment, which opens up exciting possibilities for science and technology.
What are the potential implications of your findings for the field and society?
This study reveals how cellular-like compartments can alter enzyme behavior, potentially enabling custom-designed catalysts for drug synthesis, deeper understanding of the origin of life’s chirality, and synthetic biology applications.
What was the exciting moment during your research?
The most thrilling moment was observing the complete reversal in enzyme enantioselectivity in DNA-based condensates—an unexpected and novel finding that highlights the dynamic nature of enzymes.
Paper reference: Shikha, S., Priyanka, P., & Maiti, S. (2025). Inversion of Enzymatic Enantiocatalysis Mediated by Biomolecular Condensates. Biomacromolecules. https://doi.org/10.1021/acs.biomac.5c00520
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




