Microfabricated membrane-free liver-on-a-chip for long-term culture and drug-induced liver injury modelling.
Research Summary: We developed a membrane and barrier free multicellular liver-on-a-chip that sustains long-term viability under continuous flow while precisely recapitulating liver functions for studying drug metabolism and drug-induced liver injury.
Researcher Spotlight

Preeti Sati completed her M.Tech. from the Indian Institute of Technology (IIT), Madras in Physics and currently is a Ph.D. scholar working with Prof. Sri Sivakumar at the Indian Institute of Technology (IIT), Kanpur. Her Ph.D. research work focuses on development of In-vitro liver models for closely mimicking human liver physiology and functions for studying drug metabolism, disease modelling and regenerative medicine. Her research bridges the gap between conventional cell culture models and complex behavior of the human liver.
Linkedin: https://www.linkedin.com/in/preeti-sati-16094630a/
Twitter: https://x.com/sati_preeti
Instagram: https://www.instagram.com/satipreeti/
Lab: Prof. Sri Sivakumar, IIT Kanpur
Collaborator: Prof. Sandeep Verma, IIT Kanpur
What was the core problem you aimed to solve with this research?
Liver diseases are a growing global health challenge and drug-induced liver injury is one of the leading causes of acute liver failure and a major reason for post market drug withdrawal so there is a pressing need for developing more accurate and patient-specific in-vitro liver models. However, replicating the liver’s complex architecture, especially its multiple tissue interfaces and direct cell-to-cell interactions, remains challenging as most existing liver-on-a-chip systems rely on artificial membranes or barriers that interfere in natural cell-cell communication. My research addressed this gap by developing for the very first time the novel a membrane-free liver on a chip for studying drug metabolism and drug induced liver injury that is very challenging considering to create two tissue interfaces and mimicking mass transfer similar to the actual liver without introducing artificial membrane inside the channel. This model recreates biomimetic tissue interfaces and enables more physiologically relevant natural cell interactions and mass transfer like the native liver.
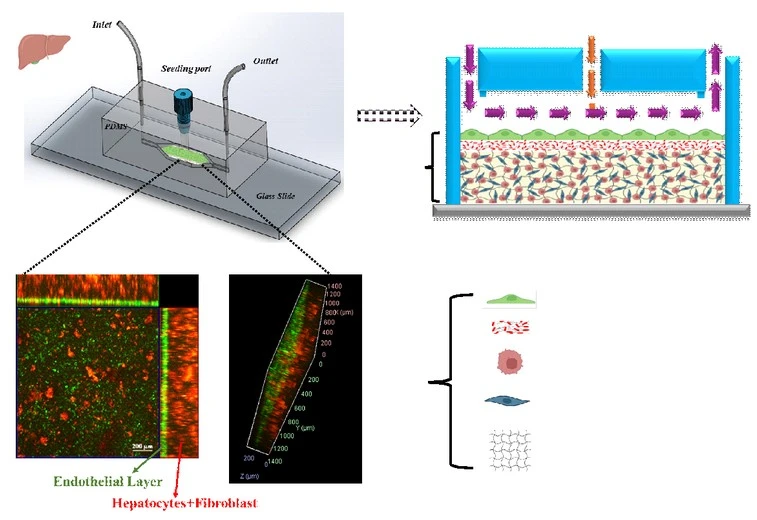
How did you go about solving this problem?
To enhance cell–cell communication among the different cell types within the chip, we pursued an alternative strategy that eliminates membranes and physical barriers. We identified PEGDA hydrogel as an effective framework for encapsulating hepatocytes and fibroblasts and found that conjugation with the bioactive peptide CGRGDS significantly improves the viability of the encapsulated cells. CGRGDS-PEGDA hydrogel not only provides 3D microenvironment to the encapsulated cells but also protects them with shear stress of flow. In addition, the hydrogel surface offers a suitable platform for establishing a stable endothelial monolayer. This architecture enables diffusion-dominated transport, preserves physiologically relevant cell–cell interactions under continuous perfusion. The unique feature in our membrane-free liver-on-a-chip lies in the elimination of artificial barriers (such as membranes and micropillars) while successfully creating two distinct tissue interfaces without compromising physiological mass transfer.
How would you explain your research outcomes (Key findings) to the non-scientific community?
In this study, we developed a miniaturized membrane-free-liver-on-a-chip that more closely mimics key structural and functional features of the human liver than conventional in vitro models. The platform supports coordinated interactions between multiple liver cell types and enables physiologically relevant transport of nutrients and drugs under continuous flow. As a result, this platform maintains stable liver-specific functions over extended periods and provides a more reliable system for studying drug metabolism drug-induced liver toxicity.
This membrane-free liver on a chip offers a robust and physiologically relevant approach for studying drug toxicity and metabolism studies. Prof. Sri Sivakumar.
What are the potential implications of your findings for the field and society?
Eliminating the artificial membrane in our system not only addresses a fundamental challenge in liver-on-a-chip platforms but also offers a scalable design framework for other organ-on-a-chip models enhancing natural tissue crosstalk and signaling dynamics. This platform is expected to significantly improve the way liver function and drug responses are studied in vitro. In addition, the system provides more in-vivo representation of human liver physiology by facilitating natural interactions between hepatocytes and endothelial cells. This leads to more accurate prediction of drug metabolism and drug-induced liver injury that help to identify toxic compounds at an early stage of drug development. The MF-LOC can reduce dependence on animal models by offering a reliable human-relevant alternative for preclinical drug screening and safety assessment. Its ability to maintain stable liver function over extended culture periods also makes it suitable for long-term toxicity studies and repeated drug exposure testing. In addition, the platform can support hepatoprotective drug evaluation and disease modeling. Overall, this innovation has the potential to lower drug development costs, improve patient safety and accelerate the translation of safer and more effective therapies to the clinic.
CGRGDS-mediated cell–matrix interactions were critical for achieving stable endothelialization under flow in our membrane-free liver-on-a-chip. Prof. Sandeep Verma
What was the exciting moment during your research?
One of the most exciting moments during my research came after encapsulating hepatocytes and fibroblasts within the CGRGDS-modified PEGDA hydrogel matrix, when we began exploring ways to establish a stable HUVEC monolayer that could withstand the shear stress generated by continuous flow. In the liver, such a steady endothelial layer regulates the diffusion of drugs and nutrients to underlying cell types. Initial attempts using conventional coatings, including Matrigel and collagen, were unsuccessful as endothelial cells detached under tangential shear stress. In contrast, functionalizing the PEGDA hydrogel surface with the CGRGDS peptide promoted strong integrin-mediated adhesion that enabled HUVEC proliferation and monolayer formation. The ability of the endothelial cells remained intact despite direct exposure to flow within the chip validated the biomimetic surface design and marked a key breakthrough in the development of the membrane-free platform.
Paper reference: P. Sati, R. Ali, M. Verma, N. Tiwari, S. Verma* and S. Sivakumar*, “Microfabricated liver-on-a-chip with membrane-free endothelial–hepatic interface for long-term culture and drug-induced liver injury modeling,” Adv. Healthcare Mater., 2026, doi: https://doi.org/10.1002/adhm.202503454
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




