Polarised MT-Nanotube with acidic vesicles renders Drosophila eggs fertile
Research Summary: These findings uncover a membrane-extended, microtubule-based nanotube in the Drosophila egg chamber that shuttles acidic/lysosomal vesicles, revealing a 3D nano-highway essential for egg fertility.
Researcher Spotlight

Sayan Acharjee is a research scholar in the laboratory of Mohit Prasad at the Indian Institute of Science Education and Research Kolkata. He completed his MSc in Biochemistry from the University of Kalyani. During his doctoral research, he focused on uncovering novel roles of cellular communication in diverse morphogenetic processes during organ development, including cell death, cell fate specification, and cell migration. He has recently submitted his PhD thesis and is now preparing to embark on the next phase of his scientific journey, driven to explore new and exciting questions.
Linkedin | Twitter | Instagram
Lab: Prof. Mohit Prasad, Indian Institute of Science Education and Research (IISER) Kolkata
Website: https://sites.google.com/view/morphogenesis-lab-iiserkolkata
Twitter: @PrasadLabIISERK https://x.com/PrasadLabIISERK
What was the core problem you aimed to solve with this research?
My senior, Dr. Banhisikha Saha, previously characterized a microtubule-based membrane protrusion that emerges from the polar cells during the late stages of oogenesis. While this discovery was intriguing, a fundamental question remained unanswered: how is this protrusion formed specifically at late oogenesis? Addressing this question proved particularly challenging, as it required the identification of signaling pathways that might facilitate protrusion formation. When I began investigating this problem, I was unable to identify any known proteins or signaling molecules within the polar cells that could account for the initiation or maintenance of the protrusion. Furthermore, downregulation of multiple candidate signaling pathways in the polar cells failed to disrupt protrusion formation, suggesting that its emergence may be regulated by other cell types.
Secondly, a broader conceptual question: if the protrusion functions to facilitate opening of the vitelline membrane for sperm entry during fertilization, what is the precise biological role and mechanistic significance of this structure? Understanding the functional relevance of this protrusion therefore remains a critical and unresolved aspect of late oogenesis.
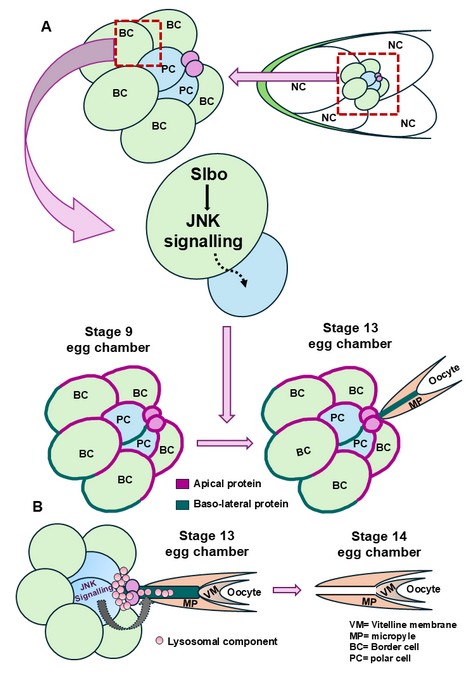
How did you go about solving this problem?
During early Drosophila oogenesis, a cluster of five to six migratory border cells, together with two centrally positioned, non-motile polar cells, migrates toward the oocyte boundary. Throughout this process, the cluster maintains a well-defined apical–basal polarity, which is essential for coordinated and directional migration. When our attempts to identify the molecular mechanisms regulating polar cell protrusion formation were unsuccessful, we shifted our focus towards a more detailed characterization of the protrusion itself. Interestingly, we observed that protrusion formation consistently initiates from apical constriction and extends toward the oocyte, irrespective of the relative positioning of the polar cells within the cluster. This observation prompted us to investigate whether the polarity of the surrounding outer border cells plays a critical role in regulating polar cell protrusion formation. Using super-resolution STED and confocal microscopy, we found that JNK signalling in the outer border cells is essential for maintaining cluster polarity, which in turn governs the initiation and orientation of the polar cell protrusion. Disruption of this polarity resulted in defects in protrusion formation, highlighting the non-cell-autonomous influence of outer border cells on polar cell behaviour.
Further ultrastructural characterization revealed that the protrusion possesses a nanotube-like architecture. Given that membrane nanotubes are known mediators of intercellular communication, we hypothesized that this structure facilitates signaling events required for opening of the vitelline membrane (VM). Since VM opening is largely regulated by localized acidic conditions, we investigated the involvement of vesicular trafficking pathways. Our data demonstrate that JNK signaling within polar cells regulates the directed transport of acidic lysosomal vesicles through the nanotubes toward the vitelline membrane. This vesicle delivery is essential for VM opening, thereby enabling sperm entry during fertilization.
“Coupling fly genetics, live cell imaging, and tissue immunochemistry, this work exemplifies the role of MT- nanotube in egg development.” – Prof. Mohit Prasad
How would you explain your research outcomes (Key findings) to the non-scientific community?
We discovered how a key step in fertilization is controlled by studying fruit fly eggs. Two specialized cells create tiny tube-like connections that act as communication bridges, sending signals to open a small gateway in the egg’s outer layer so sperm can enter. This process depends on a cellular “traffic control” system that keeps the cells correctly oriented and precisely targeted. These findings reveal a simple but powerful principle of fertility—successful reproduction relies on accurate cell communication, a mechanism that is shared across many species, including humans.
What are the potential implications of your findings for the field and society?
This discovery has important implications for the field of developmental biology and the wider community. By revealing how cells use precise orientation and direct communication to control a critical step in fertilization, the study highlights fundamental rules by which tissues organize and coordinate complex biological events. Because similar cell–cell communication and polarity mechanisms are conserved across species, these findings can inform research on fertility, early embryonic development, and developmental disorders. More broadly, understanding how cells cooperate with such accuracy may guide future studies in developmental biology, contributing to improved awareness and treatment strategies related to human development and fertility.
What was the exciting moment during your research?
The first real “aha!” moment came when we used super-resolution 3D-STED microscopy and, for the first time, clearly saw how polar cell protrusions behave. What appeared to be simple extensions were actually two protrusions merging to form a single, ultra-thin tube that stretches all the way to the egg’s protective outer layer. The second thrilling moment followed during live-cell imaging, when we watched cellular cargo actively travelling through this tiny nanotube toward the oocyte in real time. Seeing organelles move through these microscopic bridges revealed a completely new level of cell-to-cell communication during development—an exciting and unexpected mechanism that reshaped how we think about how cells coordinate complex biological events. The final and equally exciting part of this journey was working closely with all the co-authors. Their sincerity, curiosity, and constant questioning pushed the project forward at every stage, challenging assumptions and sharpening ideas. This collective drive to ask deeper scientific questions not only strengthened the work but also made the entire research process truly inspiring and rewarding.
Paper reference: Acharjee, Sayan, et al. “Polar cell membrane nanotubes containing microtubules and acidic vesicles render Drosophila eggs fertile.” PLoS biology 23.12 (2025): e3003533. https://doi.org/10.1371/journal.pbio.3003533
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




