Research Summary: The current study enlightens the glycosyl thiosulfonate as a stable electrophilic reagent, enabling efficient synthesis of thioglycosylated and thioarylation of indoles, pyrroles, and facilitating the total synthesis of bioactive Isatindigotindolosides.
Author interview

Zanjila Azeem, an SRF Fellow, is pursuing her PhD in Carbohydrate Chemistry at CSIR-Central Drug Research Institute, Lucknow, under the supervision of Dr. Pintu Kumar Mandal. Her research focuses on Stereoselective Glycosylation and C-H Activation/Functionalization of Chalcogen-glycosides synthesis.
Research Gate | Google Scholar
Lab: Dr. Pintu Kumar Mandal, AcSIR, CSIR-CDRI
Lab website: https://cdri.irins.org/profile/220155
What was the core problem you aimed to solve with this research?
In general, organosulfur compounds are known for their versatile biological activities and represent nearly 20% of all FDA-approved pharmaceuticals. Among them, thioindoles emerge as prominent structural motifs, commonly present in a wide range of biologically active natural products and therapeutic agents. Recently, a novel group of indole alkaloid glucosides, termed Isatindigotindolosides (I–IV), was discovered in Radix Isatidis, a traditional Chinese medicinal herb. These compounds have demonstrated strong anti-inflammatory and antiviral properties.
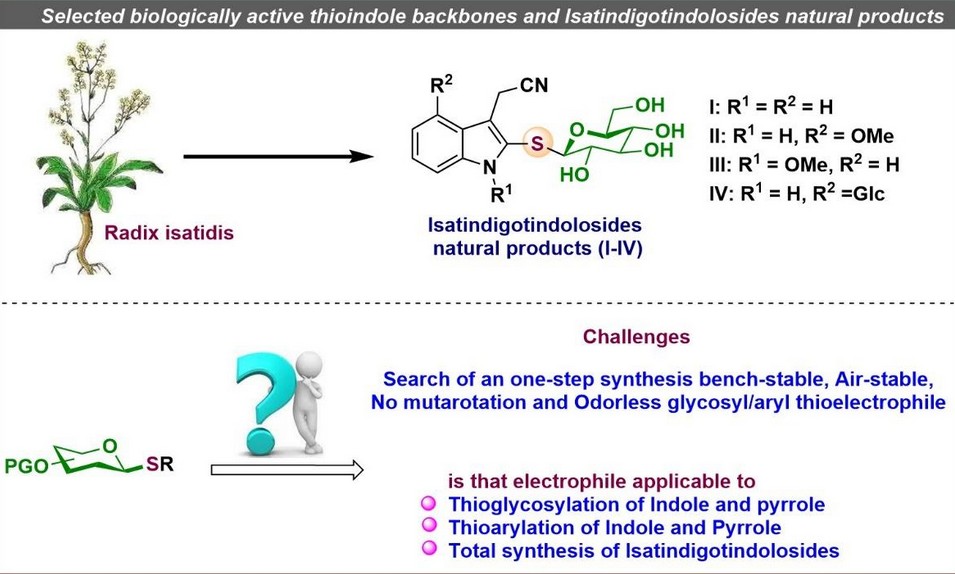
The primary challenge this research aimed to solve was the limited availability of stable and efficient electrophilic reagents for thioglycosylation, a crucial transformation in the synthesis of sulfur-linked glycosides. Traditional methods often suffer from poor stability, low reactivity, and limited functional group tolerance, making them unsuitable for the synthesis of complex molecules. This posed a significant bottleneck in the development of thioglycosylated indoles, which are valuable due to their biological relevance and presence in natural products.
How did you go about solving this problem?
To address the challenge of developing a stable and efficient method for thioglycosylation, we went through a myriad of reported literature and synthesized glycosyl thiosulfonates as novel electrophilic glycosyl donors. These reagents were chosen for their stability and reactivity under mild conditions compared to traditional glycosylation agents. Then, we systematically optimized the reaction conditions by screening various Cu catalysts, halide sources, solvents, and time to promote selective C–S bond formation between glycosyl thiosulfonates and various indole derivatives, enabling efficient synthesis of thioglycosylated indoles. The method demonstrated broad substrate scope and excellent functional group tolerance. Moreover, the utility of this approach was showcased through thioglycosylation and thioarylation of indole/pyrroles and the total synthesis of Isatindigotindolosides, a family of biologically active natural products. Overall, the research offers a robust and practical solution to longstanding challenges in sulfur glycoside synthesis.
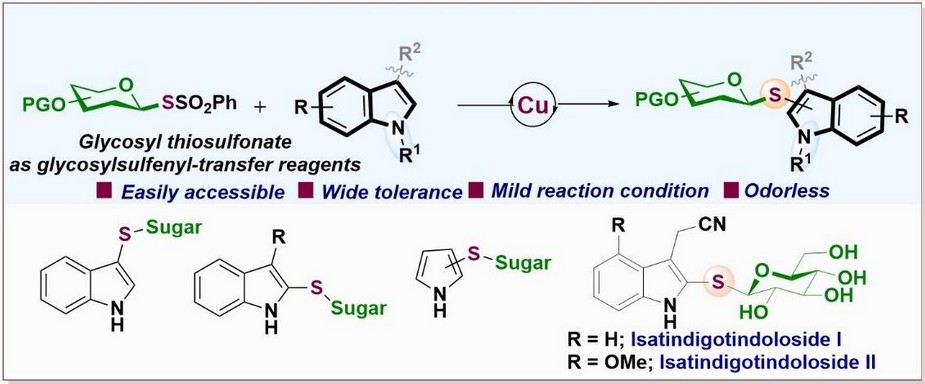
“Our findings introduce a stable reagent facilitating efficient thioglycosylation and thioarylation of indole/pyrrole, advancing access to complex bioactive molecules, Isatindigotindolosides.’’- Dr. Pintu Kumar Mandal.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Our research focused on developing a new chemical tool that helps build complex molecules more efficiently. Specifically, we utilized a stable and easy-to-handle substance called a glycosyl thiosulfonate. This compound acts like a “delivery vehicle” that can attach sugar units to other molecules in a controlled and reliable way.
Why is this important? Sugars play a vital role in how biological molecules work they affect how drugs interact with the body, how cells communicate, and how diseases develop. But attaching sugars to molecules (especially in a precise and clean way) is often difficult. In this relation, glycosyl thiosulfonate allows chemists to more easily create thioglycosides, a specific type of sugar-containing compound that’s important in drug development and natural product synthesis. Using our method, we successfully built a rare and complex natural compound called Isatindigotindoloside, found in Chinese medicinal plants, having strong anti-inflammatory and antiviral properties. This shows that our approach can be used not just in theory, but in practice to help make useful, bioactive compounds more accessible. In short, we developed a better way to build sugar-modified molecules, which could help advance medicine and chemical biology.
What are the potential implications of your findings for the field and society?
Our findings offer both scientific and societal benefits. In the scientific field, we’ve employed a stable and efficient reagent (glycosyl thiosulfonate) that simplifies the process of attaching sugars to molecules, a critical step in the synthesis of complex natural products and potential drugs. This method is safer, more reliable, and easier to use than traditional techniques, which could accelerate research in medicinal chemistry, chemical biology, and drug development.
For society, this work opens up new possibilities for the development of sugar-based therapeutics, which are often used to treat infections, cancer, and autoimmune diseases. By enabling the efficient synthesis of complex natural products like Isatindigotindolosides compounds with potential medicinal properties, our method may contribute to faster and more cost-effective drug discovery in the future. In short, our research provides a valuable tool for scientists and may ultimately help bring innovative treatments closer to reality.
What was the exciting moment during your research?
The exciting moment in our research was when we successfully utilised glycosyl thiosulfonate reagent to synthesise the complex natural product Isatindigotindoloside. This was a real turning point; not only did it confirm that our approach worked as intended, but it also showed that it could be applied to build highly intricate, biologically relevant molecules.
Seeing the final product match the natural compound’s structure after multiple precise steps was incredibly rewarding. It proved that our idea wasn’t just theoretically sound, but practically powerful, something that could be a useful tool for chemists working on real-world problems in drug discovery and synthesis. That moment validated months of hard work and opened the door to exciting future applications.
Paper reference: Azeem, Z.; Maurya, S.; Gupta, A. K.; Shalini, Kant, R.; Mandal, P. K. Glycosyl Thiosulfonate: Stable Electrophilic Reagent for Synthesis of Thioglycosylated Indoles and Total Synthesis of Isatindigotindolosides. Chem. Commun., 2025, 61, 15634-15637. https://doi.org/10.1039/D5CC03279B
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




