Research Summary: We reveal how tissue-specific ECM accelerates amylin amyloidogenesis, triggering β-cell dysfunction and glucose imbalance—uncovering a universal matrix–amyloid axis relevant across protein misfolding diseases.

First Author: Asraf is a recent PhD graduate from the Department of Biosciences and Bioengineering at the Indian Institute of Technology Bombay (IIT Bombay). His research focuses on understanding the interplay between extracellular matrix components and amyloidogenic proteins, particularly in the context of β-cell dysfunction and type 2 diabetes. He employs a multidisciplinary approach that integrates protein biophysics, cell biology, and biomaterials engineering to model disease-relevant microenvironments and uncover mechanisms of cellular stress, amyloid formation, and tissue remodeling.
Linkedin | Twitter | Instagram
Lab: Prof. Shamik Sen (Cellular Biophysics lab) and Prof. Ashutosh Kumar (Protein NMR lab), Indian Institute of Technology Bombay
Author interview
What was the core problem you aimed to solve with this research? Type 2 diabetes mellitus (T2DM) is a progressive metabolic disorder characterized by insulin resistance, chronic hyperglycemia, and eventual pancreatic β-cell failure. A hallmark of T2DM pathology is the accumulation of islet amyloid, primarily composed of misfolded human islet amyloid polypeptide (hIAPP or amylin), which contributes to β-cell stress, dysfunction, and death. Despite this well-recognized feature, the extracellular factors that drive or modulate amyloid formation remain underexplored. Collagen, the most abundant ECM protein across tissues and a major component of the islet basement membrane, is often upregulated in diabetic conditions due to fibrosis and ECM remodeling. Its structural and biophysical properties make it a strong candidate for influencing protein aggregation dynamics.
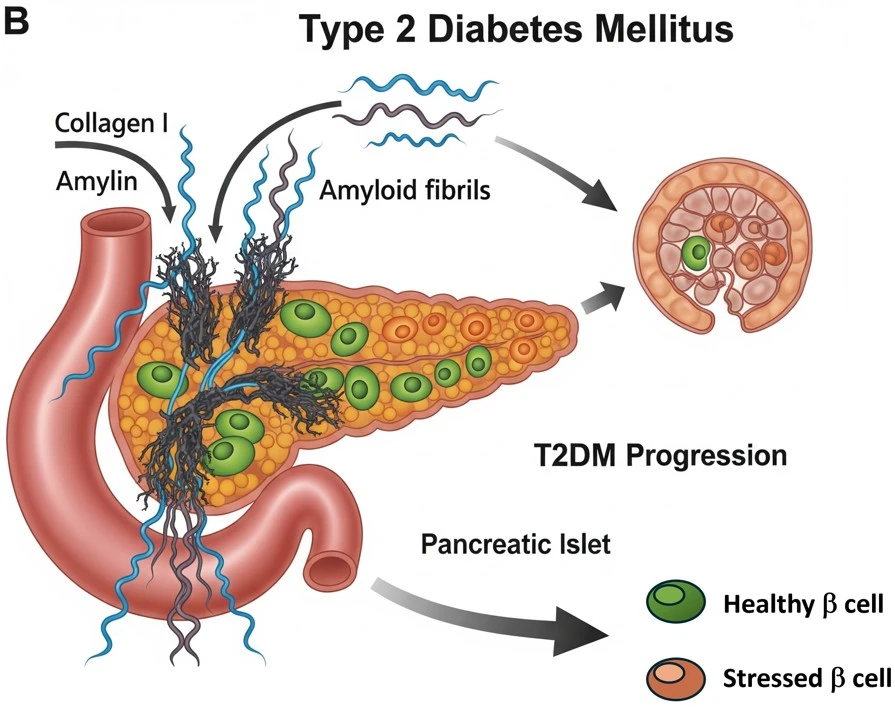
The core problem this research addresses is understanding how extracellular matrix (ECM) components, particularly collagen, influence the aggregation of amyloidogenic proteins like amylin, and how this crosstalk contributes to β-cell dysfunction and impaired glucose homeostasis in T2DM. Despite extensive research on amyloidogenic protein aggregation in diseases like T2DM, Alzheimer’s, and systemic amyloidosis, the role of the tissue microenvironment—especially ECM proteins like collagen—in modulating amyloid formation and associated cytotoxicity remains poorly understood.
Our study addresses this critical knowledge gap by modeling how collagen accelerates amylin aggregation in vitro and mimics in vivo pathological features of T2DM, such as β-cell stress and disrupted glucose regulation.
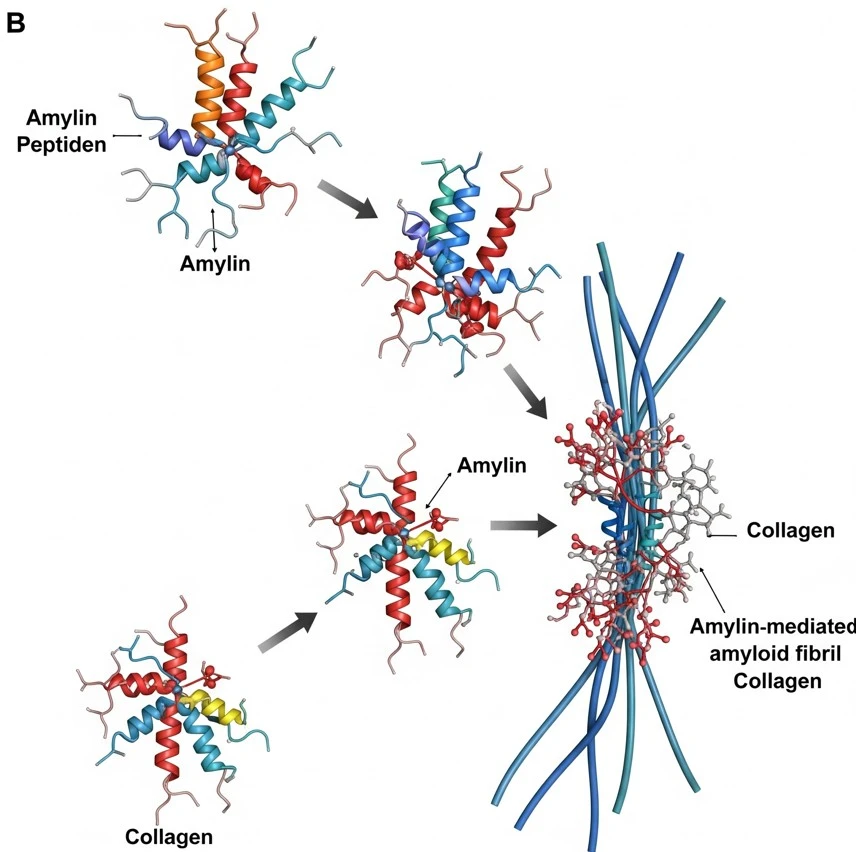
How did you go about solving this problem? To address the role of collagen in amylin aggregation and β-cell dysfunction, we developed a biomimetic in vitro system that recapitulates features of the islet microenvironment observed in type 2 diabetes. Recombinant human amylin was prepared and characterized in its monomeric form to ensure controlled aggregation kinetics. Type I collagen, a major extracellular matrix component that is upregulated in fibrotic and diabetic tissues, was incorporated into the system to examine its effect on amyloidogenesis. A range of biophysical techniques & simulations, including Thioflavin T fluorescence assays, Nuclear magnetic resonance (NMR) spectroscopy, atomic force microscopy (AFM), ,molecular dynamic simulation and circular dichroism (CD) spectroscopy, were employed to monitor the kinetics, morphology, and structural transitions of amylin aggregates formed in the presence or absence of collagen. To assess the functional impact, we exposed cultured pancreatic β-cells to these collagen-associated aggregates and evaluated cell viability, metabolic activity, insulin secretion, and markers of apoptosis. This approach enabled us to mimic key pathological features of T2DM in vitro and provided direct evidence that collagen significantly accelerates amylin aggregation and enhances β-cell toxicity. These findings underscore the importance of ECM–amyloid crosstalk as a mechanistic driver of protein misfolding and cellular dysfunction, not only in T2DM but potentially across multiple amyloid-associated diseases.
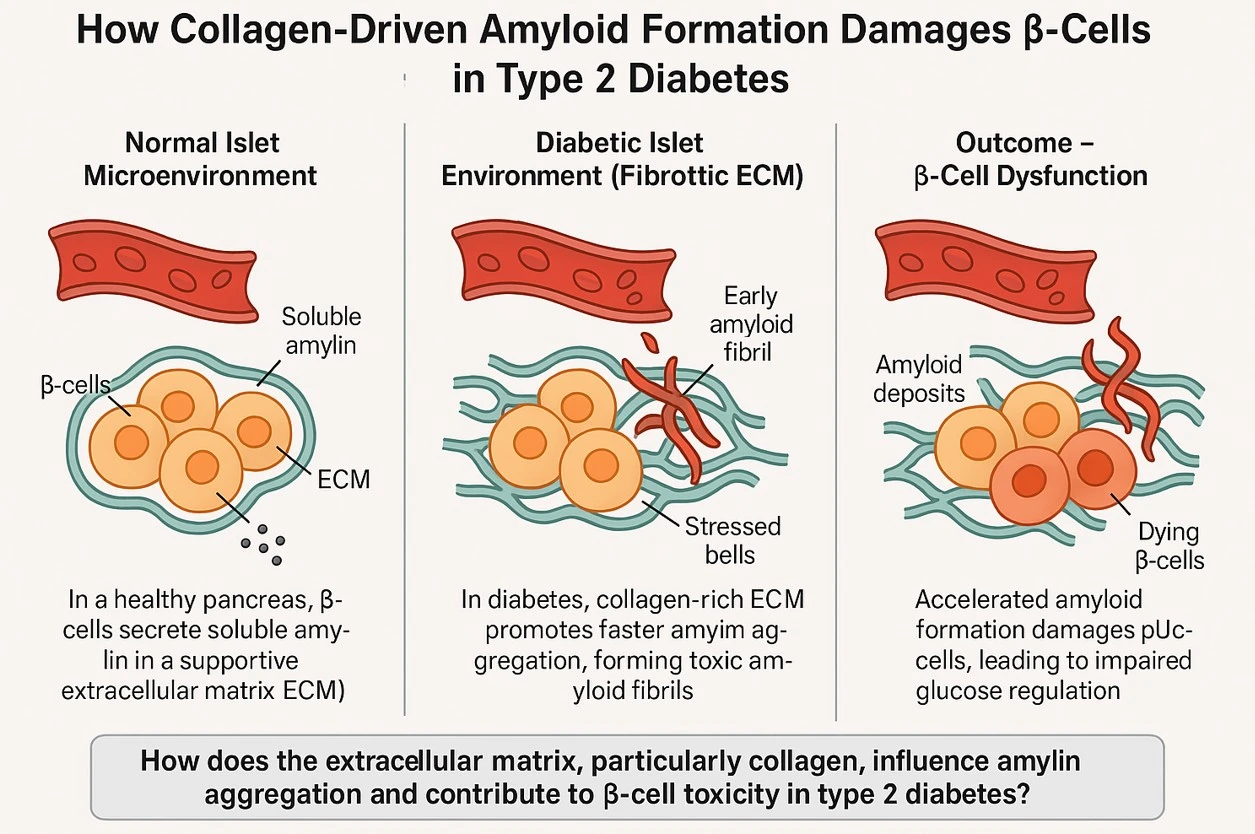
How would you explain your research outcomes (Key findings) to the non-scientific community? In type 2 diabetes, one of the major reasons the insulin-producing cells in the pancreas (called β-cells) stop working properly is because a sticky protein called amylin builds up into harmful clumps, known as amyloid. These clumps are toxic and damage the very cells that help control blood sugar.
In our research, we discovered that the supporting structure around cells—called the extracellular matrix (ECM)—plays a much bigger role in this damage than we previously thought. We focused on a common ECM protein called collagen, which is found in many tissues, including the pancreas. Using lab-grown models that mimic the environment of the pancreas, we found that collagen speeds up the clumping (or aggregation) of amylin. This leads to faster formation of toxic amyloid, which in turn causes more stress and damage to the β-cells. The result is poor blood sugar control, similar to what happens in diabetes.
This finding is important because it shows that the body’s own tissue structure can unintentionally make the disease worse, by helping harmful proteins clump more quickly. Our study helps explain how tissue changes in diabetes—and possibly other diseases like Alzheimer’s—can contribute to cell damage, and it opens the door for new treatments that target the tissue environment, not just the protein.
Fibrillar collagen I accelerates amylin aggregation, driving β-cell dysfunction and death—amplifying diabetic progression through ECM-mediated amyloid toxicity.
What are the potential implications of your findings for the field and society? I believe the findings from my research have important implications both for the scientific community and for society. By demonstrating that collagen—a major component of the extracellular matrix—can accelerate the aggregation of amylin into toxic amyloid structures, my work highlights a previously overlooked contributor to β-cell dysfunction in type 2 diabetes. This not only advances our understanding of disease mechanisms but also opens up new avenues for therapeutic intervention that go beyond targeting the misfolded protein itself. By focusing on the role of the tissue environment in promoting harmful protein aggregation, my research suggests that modulating ECM composition or structure could help delay or prevent disease progression. These insights could also be relevant to other amyloid-related disorders, such as Alzheimer’s or systemic amyloidosis, where ECM remodeling is common. On a broader scale, this work could contribute to the development of safer biomaterials for regenerative medicine and islet transplantation, as well as early diagnostic tools based on ECM alterations—ultimately leading to more effective, tissue-informed strategies to manage and treat chronic diseases.
What was the exciting moment during your research? One of the most exciting moments in my research was watching how quickly amylin, a protein linked to diabetes, started forming harmful clumps when collagen was added. Under the microscope, I could see these dense, thread-like amyloid structures forming much faster than expected. It was a powerful moment—realizing that the tissue structure itself could speed up disease-related damage. That visual confirmation brought everything together and made it clear that the environment around cells, not just the proteins inside them, plays a huge role in how diseases like type 2 diabetes develop. It was both thrilling and eye-opening.
Paper reference: https://pubs.acs.org/doi/10.1021/jacs.4c15698
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!