Research Summary: We discovered a redox switch in drug-tolerant Triple-Negative Breast Cancer (TNBC) persister cells: GPX4 suppression triggers FSP1 upregulation, promoting chemoresistance. Co-targeting GPX4–FSP1 selectively kills drug-tolerant cells by inducing ferroptosis.
Author interview

Dr. Nazia Chaudhary is a cancer biologist at ACTREC, Tata Memorial Centre, focusing on therapy resistance, ferroptosis, and redox metabolism in aggressive breast cancers.
LinkedIn https://actrec.gov.in/dr-nazia-chaudhary
Twitter @Chaudhary9Nazia

Ms. Dibita Mandal is a project fellow working under an international DST-CEFIPRA project at ACTREC, Tata Memorial Centre, focusing on therapeutic potential of ferroptosis as Triple-Negative Breast Cancers.
LinkedIn: www.linkedin.com/in/dibita-mandal-5ab208201
Lab: Dr Nandini Verma, Advanced Centre for Treatment, Research, and Education in Cancer (ACTREC), Tata Memorial Centre, Navi Mumbai, India
Twitter/X– @DrVermaNandini, @TNBCL
What was the core problem you aimed to solve with this research?
Chemotherapy is the main therapeutic approach for TNBC, an aggressive subtype of breast cancer for which there are no specific targeted treatment alternatives available. More than half of TNBC patients do not respond well to chemotherapy or have early relapse with highly resistant and metastatic cancer. These tumor cells are also known to be resistant to apoptotic cell death. How to eliminate the aggressive and chemo-tolerant population of TNBC cells is a big clinical challenge, as we do not understand enough about the common vulnerability of these TNBC zombie cells, which comes from heterogeneous molecular backgrounds. Moreover, how to recognize this type of stubborn disease cells in patients, we do not have any special molecular signatures yet.
How did you go about solving this problem?
Through our previous work, we identified that TNBC cells are intrinsically more susceptible to iron-mediated redox cell death called “ferroptosis” due to their molecular makeup (Verma et al. 2020, Science Advances).
We asked a broad question: whether the ferroptosis susceptibility of TNBC can be utilized to devise a method to target chemo-resistant cells that are intrinsically resistant to apoptosis.
Therefore, we started primarily by trying to understand what happens to the susceptibility to ferroptosis in TNBC cells when they acquire chemotherapy-tolerance.
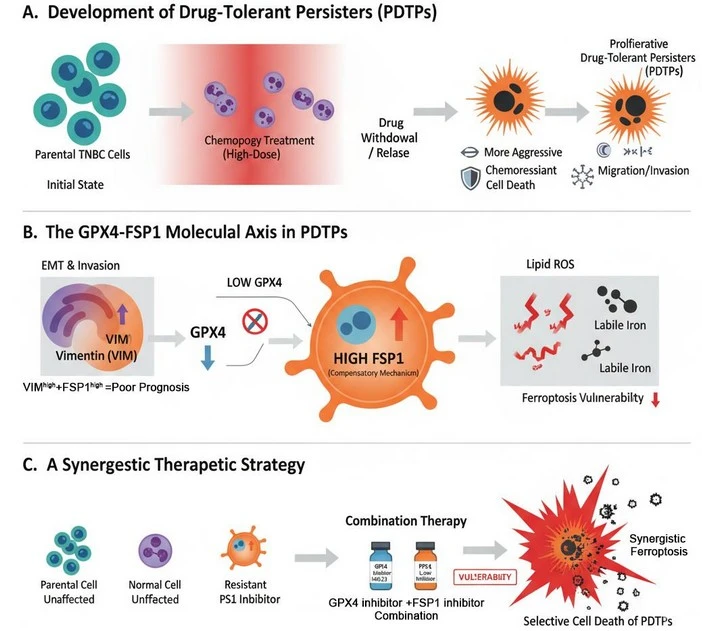
To answer this question, we developed cellular models of longitudinal drug resistance by deriving Drug-Tolerant Persister (DTP) Cells from different molecular subtypes of TNBC and characterized their cellular and molecular features. DTP cells are a relatively low population of cancer cells that can survive high doses of chemotherapy, go latent, and then resurfaces as proliferative, drug-resistant populations. Our early results showed that the DTP populations of TNBC are far more aggressive in terms of growth and have much higher migratory and invasive behavior as compared to their parental cell population, which aligns with the clinical observations in patients. We next analyzed the fragility of these DTP cells to known ferroptosis-inducing agents, together with other critical factors that control it, like cellular amounts of redox-active iron pools (Fe2+), cellular redox enzymes, and their regulators in the ferroptosis pathway. We found that DTP cells were highly susceptible to ferroptosis-inducing agents due to a dramatic downregulation of the central lipid-ROS managing enzyme, Glutathione peroxidase 4 (GPX4), which maintains the reduced form of Glutathione (GSH) in these cells, coupled with increased cellular lipids and Fe2+- a perfect recipe for ferroptosis said Dr. Nazia.
These results were encouraging, as we knew they could pave the way for ferroptosis-mediated therapeutic approaches for TNBC. However, one question always struck us back and made us think that there is something beyond it, which we are missing. That is because, despite dramatically suppressed GSH pathway (due to decreased GPX4 and master transcription factor NRF2) and high active iron in these cells, they are not just surviving but also happily proliferating without any sign of stress, until treated with ferroptosis inducers. Interestingly, we found that the DTP cells are able to do it because they very smartly made a switch to another ferroptosis inhibiting enzyme called Ferroptosis Inhibiting Protein-1 (FSP1) by abruptly increasing its level, an enzyme that utilizes not GSH but Coenzyme Q10 (Ubiquinol) as a co-factor, Ms. Dibita Mandal explained with excitement; who is the second author of the study involved in the discovery of GPX4-FSP1 switch and execution of the targeting part. This was dramatic, and more interestingly we found that GPX4 itself regulates the upregulation of FSP1, experimental modulation of GPX4 expression (genetic, chemical or pharmacological) will result in sharp upregulation of FSP1 in all subtypes of TNBC cells. It is worth mentioning that several types of chemotherapies could indirectly put this switch “ON” by inducing an acute downregulation of GPX4 within 48 hours of treatment.
Curiously, we wanted to understand that if we hit this sneaky survival Redox shift what will be the fate of the TNBC DTP cells. The answer was even more encouraging; yes, these cells succumbed to ferroptosis if you stop both GPX4 and FSP1 together. We show that it is possible to target these adaptive mechanisms therapeutically.
We also established that GPX4low/FSP1high + VIMhigh expression is a strong molecular signature for TNBC DTPs, and is significantly associated with poor prognosis in patients as well. Therefore, these findings are clinically relevant as we can identify this notorious TNBC DTP population in patients using this molecular signature and therefore it has a potentially high theranostic value.
“Our findings redefine therapy resistance, exposing a hidden blueprint in persister cells’ that could transform treatment of the most stubborn breast cancer and beyond.” –Dr. Nandini Verma
How would you explain your research outcomes (Key findings) to the non-scientific community?
This study looked at why triple-negative breast cancer (TNBC), one of the most aggressive breast cancers, often survives and comes back after chemotherapy. This disease attacks the mostly younger population of women and its prevalence in India is almost double of what we see in the western countries. While chemotherapy kills most cancer cells, some tough nuts survive in a “sleeping” state called drug-tolerant persister (DTP) cells. These cells later “wake up,” and grow again, and spread, causing relapse.
We capture this phenomenon in petri-dishes in the lab to study the cell and molecular biology of these DTPs to understand what could happen in the clinic in the tumors of TNBC patients. We found that these survivor TNBC DTP cells don’t just lie dormant. They change shape and behavior, becoming more mobile and invasive, features, which help them to grow fast and spread to other parts of the body. They also carry damaged mitochondria (the cell’s powerhouses), build up harmful molecules called Reactive Oxygen Species (ROS), and store extra iron, which makes them vulnerable to a special type of cell death called ferroptosis.
Two key proteins play a central role. GPX4, a protective enzyme, is found at very low levels in these cells, and its loss is linked to worse patient outcomes. At the same time, another protein, FSP1, rises sharply, helping cancer cells survive chemotherapy and resist further treatment.
The most important finding is that blocking both GPX4 and FSP1 together kills these stubborn cells while sparing normal breast cells. This reveals a potential new treatment strategy to prevent relapse and improve survival for TNBC patients.
What are the potential implications of your findings for the field and society?
Our study provides important insights into one of the biggest challenges in TNBC: the persistence of chemotherapy-resistant cells that fuel relapse and metastasis. Although TNBC cells are intrinsically more susceptible to ferroptosis compared to non-TNBC subtypes, we show that drug-tolerant persister cells survive by undergoing a dramatic redox reprogramming — switching from GPX4-dependent glutathione metabolism to FSP1-mediated ubiquinone reduction. This adaptive shift fulfills the heightened metabolic demands of aggressive persister cells, but at the same time, it exposes a previously unrecognized therapeutic vulnerability.
From a translational perspective, this work has three major implications. First, it explains why TNBC patients, despite initial chemotherapy responses, frequently relapse with tumors that are more aggressive — the GPX4–FSP1 redox switch underlies persistent survival. Second, it identifies a prognostic signature (GPX4low, FSP1high, and VIMhigh) that is therapeutically useful for stratifying patients who are most at risk of resistance and metastasis and has a substantial correlation with poor survival. Third, it draws attention to a new treatment approach: dual inhibition of GPX4 and FSP1 can kill persister cells only while sparing healthy cells, either alone or in conjunction with chemotherapy or ferroptosis inducers.
Beyond TNBC, these findings are likely to have broader relevance, as ferroptosis regulators are increasingly recognized as critical determinants of drug resistance across solid tumors. In this way, our study not only explains relapse in TNBC but also lays the foundation for biomarker-driven precision medicine and rational drug combinations that could significantly improve outcomes for patients with aggressive cancers.
Our study opens the door to smarter, more personalized treatments for hard-to-treat breast cancers, offering both a scientific breakthrough and real societal value by improving survival and reducing relapse.
What was the exciting moment during your research?
The finding that persister cells survived chemotherapy by significantly upregulating FSP1 despite exhibiting all the characteristics of ferroptosis vulnerability, including high iron levels, poor glutathione, and mitochondrial damage was very intriguing- Nazia with Dibita shared their exciting experience of developing this project in this direction.
This demonstrated a redox change from GPX4 to FSP1, a hidden metabolic adaptation. This modification was significant because it revealed a therapeutic vulnerability that could now be addressed in addition to explaining persister cell survival.
Paper referenc: https://www.sciencedirect.com/science/article/pii/S2213231725003775?via%3Dihub
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




