Research Summary: The current study reports on a FRET based structural probe to quantify folded back or OFF state of β-cardiac myosin, a motor protein responsible for contraction of human heart.
Author interview

Dr. Rama Reddy Goluguri is a Basic Life Research Scientist in Prof. James Spudich/ Kathy Ruppel’s lab at Stanford University, studying β-cardiac myosin and how disease-causing mutations alter its structure and function.
Linkedin: Rama Reddy Goluguri
Twitter: goluguri@8
Lab: Prof. James Spudich and Prof. Kathleen Ruppel, Stanford University, USA
What was the core problem you aimed to solve with this research?
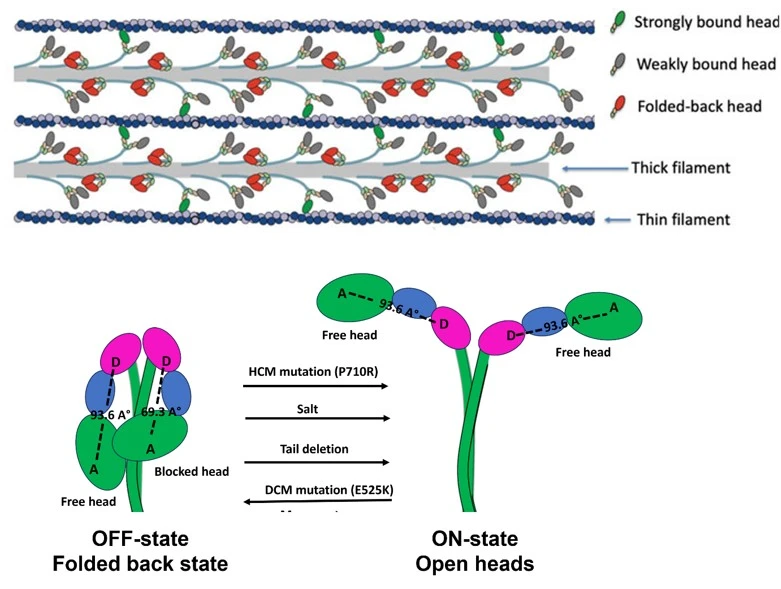
In muscle cells, force is generated by the sliding of actin-containing thin filaments over myosin-containing thick filaments. However, not all myosin motor heads participate in force production at any given time. A subset of myosin molecules adopts a structural conformation known as the interacting-heads motif (IHM), also referred to as the folded-back or OFF state. These OFF states are critical for the regulation of muscle contractility and are known to be disrupted in several cardiomyopathies.
Recent advances in structural biology have provided exquisite near atomic-level details of the folded-back state within thick filaments. However, these static structures cannot capture the dynamics of how OFF states form or how they are switched back to the active state when needed. To probe these dynamics, several labs, including ours, have used a biochemical single-turnover assay that monitors single ATP turnover by myosin with a fluorescent ATP probe. The fraction of myosin heads that hydrolyze ATP extremely slowly has been termed the super-relaxed (SRX) state. Yet, evidence from our lab and others indicates that the single ATP turnover assay does not reliably report on the OFF states of myosin.
The main objective of this study is therefore to develop a structure-based assay that can directly and quantitatively measure the OFF states of myosin in solution.
How did you go about solving this problem?
Fluorescence Resonance Energy Transfer (FRET) is the method of choice for probing myosin regulation because of its ability to measure nanometer-scale distances and capture dynamic conformational changes. While there have been previous attempts to design FRET sensors to quantify the OFF states of myosin, our work was uniquely enabled by the availability of the atomic structure of the folded-back state of human β-cardiac myosin, which provided a precise blueprint for sensor design.
In addition, our team has established methodologies to express and purify human β-cardiac myosin, both the wildtype protein and multiple variants containing disease-causing mutations. Combined with the wealth of biochemical data on mutant variants of myosin, this positioned us ideally to address the challenge. Through carefully designed experiments and a strategic collaboration with Prof. Dave Thomas’s lab at the University of Minnesota, we successfully developed a FRET assay that reliably reports on the OFF states of myosin.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Every heartbeat is driven by a tiny motor protein called β-cardiac myosin in heart muscle. To pump the right amount of blood, whether at rest or during exercise, the heart must carefully control how many of these motors are active. Myosin can switch between an ON state (active, generating force) and an OFF state (inactive, resting).
Until now, scientists had no direct way to measure how much myosin is in the OFF state compared to the ON state. In this study, we developed a new method that can detect and measure the OFF state. We also used this method to understand how myosin switches from the active ON state into the resting OFF state.
“The rapid kinetics of formation of the OFF-state of cardiac myosin described here is fundamental to cardiac function” says Prof. James Spudich, The Douglass M. and Nola Leishman Professor of Biochemistry and of Cardiovascular Disease at Stanford University
What are the potential implications of your findings for the field and society?
Historically, muscle contraction was understood to be regulated by calcium signaling, which displaces troponin and tropomyosin from actin-containing thin filaments. This displacement allows cross-bridge formation between myosin heads in the thick filament and actin, resulting in force production. More recently, it has become clear that thick filaments also contribute to the regulation of contractility by modulating the number of myosin heads available to interact with actin. This thick-filament–based regulation is achieved through the IHM or the folded-back state of myosin. In the IHM, the catalytic heads of myosin fold back onto its coiled-coil tail, sequestering them away from actin.
Disruption of the folded-back state, leading to an increased number of myosin heads participating in contraction, has been proposed by our lab as a unifying mechanism for the pathogenicity of many hypertrophic cardiomyopathy (HCM) mutations in myosin. However, there is no direct assay to quantify the IHM state, and the mechanisms underlying its formation and disruption remain poorly understood.
To address this, we developed a FRET-based sensor that reliably quantifies the IHM state. Using this sensor, we identified critical steps during IHM formation.Our sensor not only enables mechanistic insight into thick-filament–based regulation at the molecular level but also provides a valuable tool to evaluate drugs that stabilize or destabilize the IHM state.
What was the exciting moment during your research?
The most exciting moment was when I finally developed a kinetic model that explained the data, after a week of thinking and carefully reading relevant papers. Interpreting the kinetic data was initially a major challenge. At first, we could not make sense of the results, so we took a systematic approach: asking very basic questions, examining different conformational states of myosin to identify which ones were sensitive to FRET, and studying previous work on myosin kinetics. Careful reading of papers on ATP binding and the associated structural changes in myosin, along with discussions with Prof. Mike Geeves, an expert in myosin kinetics, helped us refine our understanding. Ultimately, this process led us to a model that quantitatively explained our results.
Paper reference: Goluguri, R. R., Guhathakurta, P., Nandwani, N., Dawood, A., Yokota, S., Roopnarine, O., Thomas, D. D., Ruppel, K. M., & Spudich, J. A. (2025). A FRET assay to monitor different structural states of human β-cardiac myosin including the interacting-heads motif. Proceedings of the National Academy of Sciences, 122(34), e2504562122. https://www.pnas.org/doi/10.1073/pnas.2504562122
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




