Research Summary: Vitamin B12 alters the methionine cycle in C. elegans serotonergic neurons, triggering serotonin-neuropeptide signaling that rewires neuron-gut communication, enhancing stress resilience, foraging behavior, and longevity.
Author interview

Dr. Sabnam Sahin Rahman completed her PhD at the National Institute of Immunology, New Delhi, under the supervision of Dr. Arnab Mukhopadhyay, where she elucidated how neuron-gut signaling mediates the effects of a micronutrient-driven diet-gene pair in regulating longevity.
Twitter: https://x.com/SABNAMRAHMAN3
Lab: Dr. Arnab Mukhopadhyay, National Institute of Immunology, New Delhi, India
Twitter: https://x.com/smartelegans
What was the core problem you aimed to solve with this research?
Vitamin B12 (B12) is an essential micronutrient involved in fundamental metabolic pathways, particularly the folate and methionine cycles. While B12 deficiency is clinically linked to neurological disorders and depression the molecular mechanisms through which it influences body-wide physiology, especially across tissue boundaries, are still elusive.
We asked a central question: Can a micronutrient like B12, sensed in one tissue, orchestrate changes in distant organs to regulate essential processes like stress response, behavior, and even longevity?
Our goal was to explore whether B12 could act as a nutritional signal, linking diet to inter-tissue communication and whole-body adaptation.
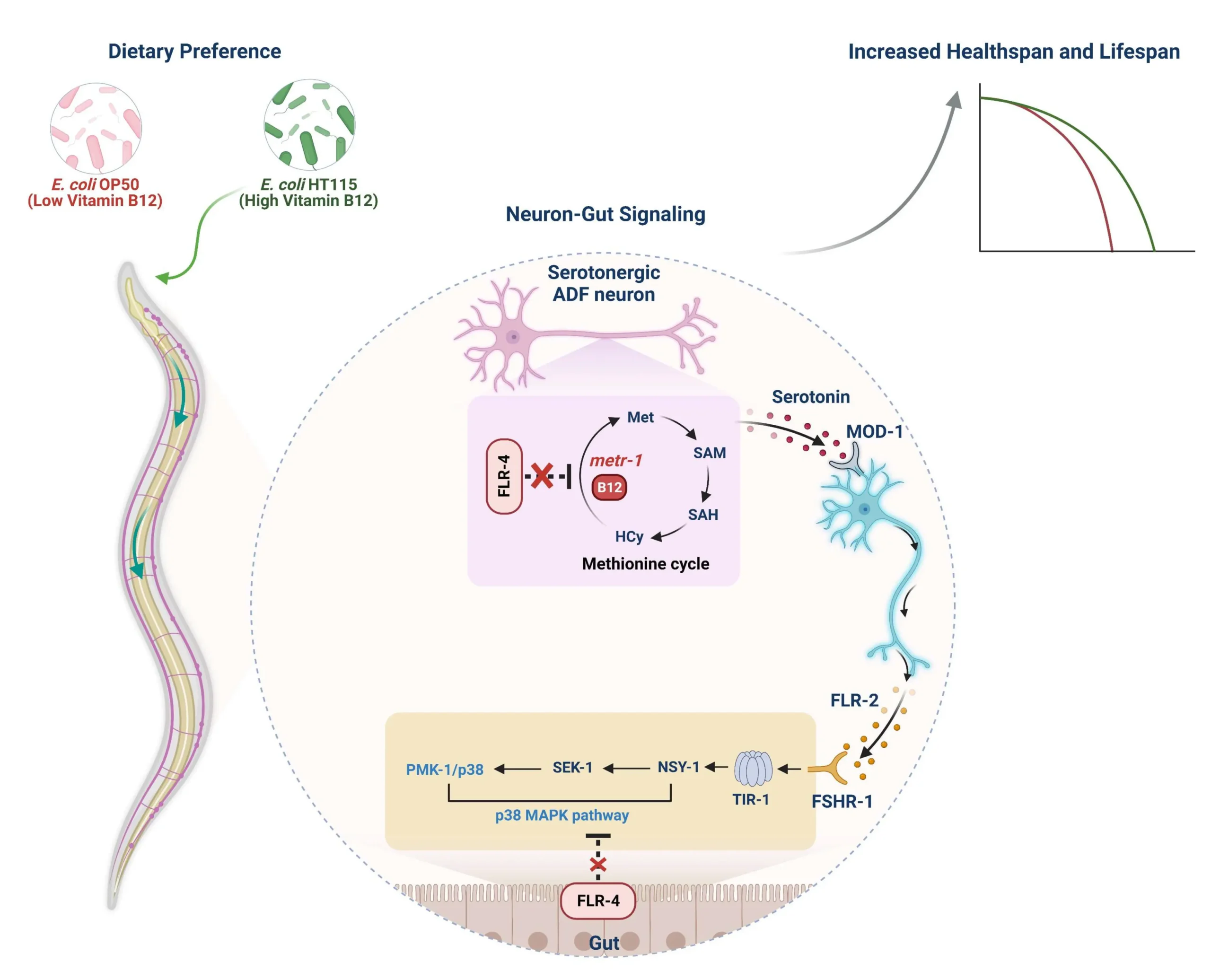
A model delineating the mechanism by which C. elegans FLR-4 modulates longevity through a neuron-gut crosstalk, preventing aberrant activation of the Met-C and the p38-MAPK pathway on a diet containing different B12 levels. In the flr-4 mutant, grown on a high B12 diet, the flux through the Met-C localized in the ADF serotonergic neurons directs the release of serotonin, which binds to MOD-1, its cognate receptor in the interneurons. These interneurons, in turn, release the neuropeptide FLR-2, which then binds to its cognate receptor FSHR-1 in the intestine. This cascade induces the oligomerization of TIR-1, activating the downstream p38-MAPK pathway. Consequently, this leads to an increase in CyTP gene expression, enhancement of osmotic stress tolerance, and an extension of the lifespan of the flr-4 mutant grown on a high B12 diet. The worms with a normal version of FLR-4 will prevent a high B12 diet from altering their life history traits, giving it resilience that is required to grow in their environmental niche.
How did you go about solving this problem?
We used the tiny roundworm Caenorhabditis elegans as our model organism, known for its genetic simplicity and well-mapped nervous system. Our focus was on a kinase-dead mutant of the flr-4 gene, which showed heightened sensitivity to B12 in the diet.
Using an integrative approach, RNA interference, tissue-specific gene rescue, metabolomics, fluorescence imaging, behavioural tracking, and lifespan assays, we uncovered that dietary B12 alters the methionine cycle (Met-C) specifically within ADF serotonergic chemosensory neurons. This metabolic rewiring enhances serotonin synthesis via enhanced transcription of tph-1, the gene encoding tryptophan hydroxylase.
The elevated serotonin signal acts on downstream interneurons through the MOD-1 receptor, triggering the release of the neuropeptide FLR-2. FLR-2 then binds to its receptor, FSHR-1, on intestinal epithelial cells. This engagement triggers a phase transition of TIR-1, the C. elegans ortholog of mammalian SARM1, culminating in the activation of the innate immune p38-MAPK pathway. The result is an upregulation of cytoprotective gene expression, enhanced stress tolerance, and extended lifespan.
Intriguingly, this neuron-gut axis also reshapes foraging behavior. Worms begin to preferentially seek out B12-rich bacterial diets, suggesting that this neural-metabolic circuitry not only boosts survival but also reinforces dietary choices that promote longevity.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Imagine if our brain could sense what we are eating and then instruct our gut, not just to digest the food, but to switch on internal defenses that help us live longer. That is essentially what we discovered, using tiny transparent worms as our model.
We observed that when these worms consume food rich in vitamin B12, a specific pair of neurons in their brain recognizes it and responds by synthesizing more serotonin, the same chemical that boosts human mood and well-being. This serotonin acts as a messenger, signaling downstream neurons, which then directs the intestine to activate stress-defense genes.
But that’s not all. This brain-gut conversation also changes behavior; the worms begin to prefer B12-rich foods, almost as if their brain remembers or is aware that such food will help them survive better.
Though we discovered this in worms, the concept has deep implications. It shows that food isn’t just about calories/fuel, it’s information. Nutrients like B12 can shape how our nervous and digestive systems talk to each other, influencing how we behave, adapt, and age.
What are the potential implications of your findings for the field and society?
Our study reveals an elegant molecular framework through which dietary nutrients, specifically, microbiota (our gut resident beneficial bacteria)-derived vitamin B12, can modulate brain function and, in turn, reshape systemic physiology. We demonstrate that B12 is not just a metabolic cofactor but acts as a potent signaling molecule that, when sensed by specific neurons, triggers a series of events that influence distant tissues, particularly the gut, resulting in improved stress resilience, altered food-seeking behavior, and extended lifespan.
For the scientific community, especially in the fields of aging, neurobiology, and metabolism, our findings provide a framework to understand how neurons act as nutrient sensors. By mapping a serotonin-based neuron-gut signaling axis that is driven by B12-dependent changes in the methionine cycle (a metabolic hub), we establish a direct link between dietary inputs, neural metabolism, and whole-body adaptation. This opens new avenues to explore how individual neurons can coordinate broad physiological programs and how nutrient-sensing mechanisms may be conserved across species.
The broader societal implications are equally profound. In recent years, conditions like age-related cognitive decline, mood disorders, and chronic gut dysfunction have been increasingly linked to poor diet and imbalances in the gut microbiome. Our work provides a mechanistic bridge between diet, microbiota, and neural circuits, demonstrating how a nutrient such as B12 can influence not only metabolic health but also behavior, resilience to stress, and longevity. These insights offer exciting opportunities to design targeted nutritional or microbial interventions to delay aging, boost mental health, or treat disorders rooted in brain-gut miscommunication.
Importantly, this study also strengthens the emerging view that food is more than a source of calories; it may be a form of molecular communication. Nutrients can serve as informational cues that recalibrate how the brain and gut interact, ultimately shaping how organisms respond to their environment and how they age.
“This work uncovers a novel micronutrient-driven brain-gut signaling axis that coordinates stress resilience, behavior, and longevity.”
What was the exciting moment during your research?
The most unforgettable moment came when we found that restoring methionine cycle, a key cellular metabolic hub, function in just one pair of serotonergic neurons, the ADF chemosensory neurons, was sufficient to rescue the entire systemic phenotype: gut stress tolerance, behaviour, and extended lifespan.
It was a wonderful illustration of biological elegance how the effects of reprogramming the metabolism of two neurons could ripple across the entire body, coordinating stress defenses and longevity through a cascade of neural and endocrine signals. Watching serotonin levels surge, intestinal cytoprotective reporter glow, and behavior rewire, all from a tiny change in neural metabolism, was an awe-inspiring realization of the power of inter-tissue communication.
Paper reference: Rahman, S.S., Bhattacharjee, S., Motwani, S. et al. Methionine cycle in C. elegans serotonergic neurons regulates diet-dependent behaviour and longevity through neuron-gut signaling. Nat Commun 16, 5118 (2025). https://www.nature.com/articles/s41467-025-60475-0
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




