🔔 New Online Course Launch – Enroll Now! 🔔
🎓 Course Title:
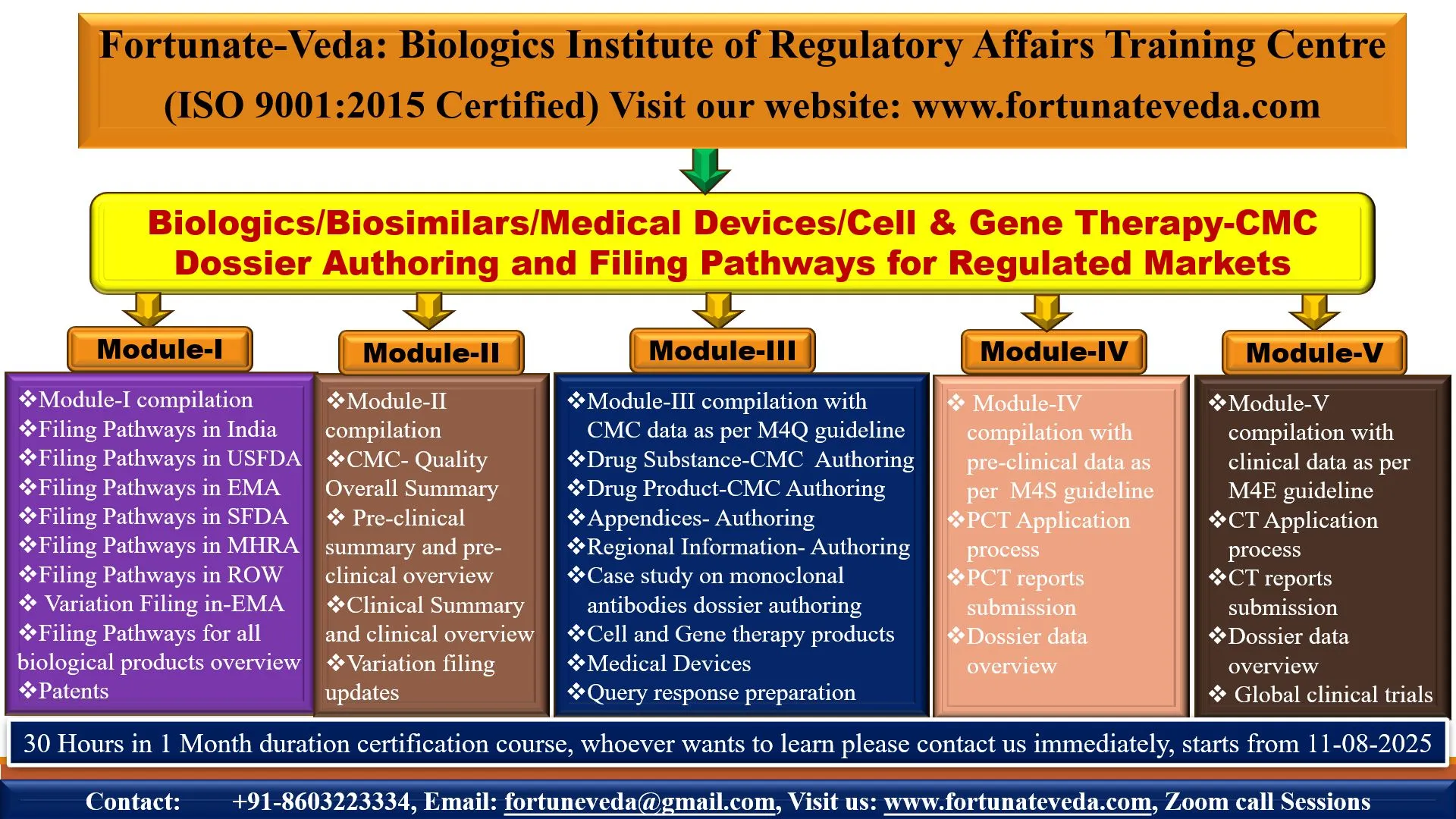
Diploma in Drug Regulatory Affairs CTD/ACTD/eCTD Dossiers Preparation & Global Submission Strategies for Biologics, Biosimilars & Vaccines
📅 Start Date: 11th August 2025
🕘 Time: 9:00 PM – 10:30 PM IST
📚 Total Duration: 30 Hours
💻 Mode: Online (Zoom Call Sessions)
📌 Course Highlights:
✅ CTD structure (Modules 1–5) with region-specific insights
✅ Dossier preparation for USFDA, EMA, WHO, ROW markets and CDSCO
✅ Regulatory & CMC documentation best practices
✅ eCTD submission strategies and case studies
✅ Ideal for Regulatory Affairs, CMC, QA/QC & RA professionals
📞 For Registration & Details:
📱 +91-8603223334
🌐 www.fortunateveda.com
✉️ Email:
fortuneveda@gmail.com
contact@fortunateveda.com
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!




