Axonal Pruning Reveals a Tipping Point in Brain Aging
Research Summary: We integrate aging-related, small-vessel-driven white-matter lesion burden to map brain aging and health, identifying a threshold beyond which axonal fiber loss accelerates structural and cognitive decline.
Researcher Spotlight

Niraj Kumar Gupta is a PhD scholar in the Neuroimaging and Brain Biology Group of Dr. Vivek Tiwari, Dept of Biological Sciences, Indian Institute of Science Education and Research (IISER) Berhampur. He seeks to understand why aging erodes physiological efficiency and cognition. His research integrates cerebrovascular pathology, white-matter integrity, and method development with advanced neuroimaging to uncover mechanisms driving divergent brain-aging trajectories and to redefine “healthy” aging.
LinkedIn: https://www.linkedin.com/in/nirajgupta11/
Twitter: https://x.com/nirajgupta02
Lab: Dr. Vivek Tiwari, PhD, Assistant Professor, Department of Biological Sciences, Indian Institute of Science Education and Research (IISER) Berhampur, India 760003
Profile: https://iiserbpr.ac.in/people/profile/vivekt
PI’s social media: https://x.com/iamvivekt , https://www.linkedin.com/in/vivek-tiwari-76b73087/
Lab social media: X: https://x.com/nibbrlab
Lab webpage: https://sites.google.com/iiserbpr.ac.in/nibbrlab/home
What was the core problem you aimed to solve with this research?
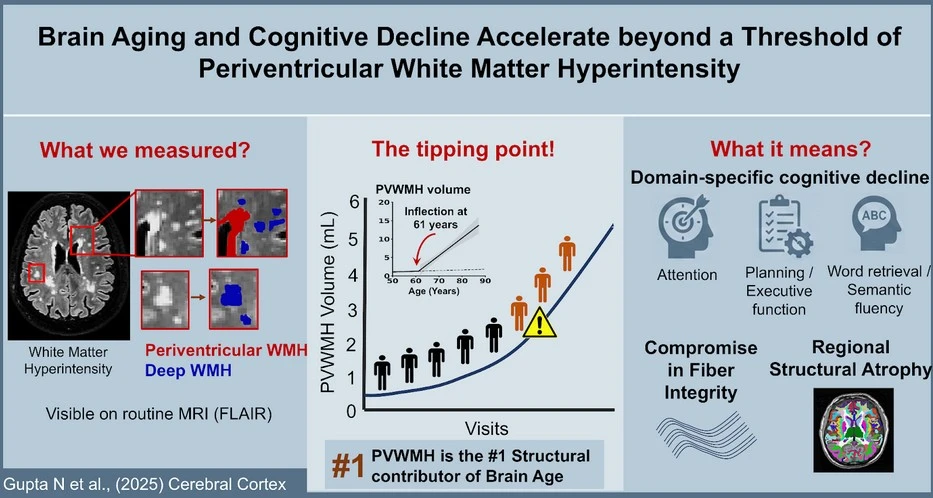
As we age, everyone experiences small-vessel-related injury in the brain that gradually prunes white-matter fibers essential for neural communication. However, this process is highly variable. Some individuals show minimal fiber loss, others moderate changes, while a subset undergoes excessive pruning. The core problem we addressed was identifying when this universal aging process becomes biologically disruptive. Our study shows that once small-vessel–driven white-matter injury crosses a critical threshold, the brain enters a phase of accelerated structural loss and selective cognitive decline, even in people still considered cognitively normal. This reveals brain aging as a heterogeneous, threshold-driven process rather than a uniform, gradual decline.
- How did you go about solving this problem?
To address these questions, we combined data from our Brain Imaging Aging cohort at IISER Berhampur with large global aging cohorts, including the National Alzheimer’s Coordinating Center and Alzheimer’s Disease Neuromaging Initiative. We implemented in-house developed methods to segment white-matter hyperintensities, separately quantifying periventricular and deep white-matter lesions. These measurements were then integrated with longitudinal, aging-focused neuroimaging analyses of brain volumetry and with domain-specific cognitive behaviors. This multiscale approach allowed us to relate regional white-matter injury to progressive changes in brain structure and cognition across the adult lifespan, and to distinguish resilient from accelerated brain-aging trajectories.
How would you explain your research outcomes (Key findings) to the non-scientific community?
As we age, the brain’s communication pathways – the “wiring” that allows different regions to talk to each other – gradually wear down. This happens to everyone, but not at the same pace. Some people experience relatively slow changes, while others show much faster breakdown of these nerve fibers, which is linked to poorer brain health.
In our research, we identified a clear tipping point. When this wiring damage – seen as white-matter lesions – crosses a modest volume (about half a teaspoon; ~2.3 mL), the brain begins to age more rapidly. Beyond this point, people are more likely to lose brain tissue and develop difficulties with everyday mental skills such as attention, planning, multitasking, and finding the right words. Importantly, these changes can occur even when standard memory tests still appear normal.
We also found that not all white-matter damage behaves the same way. Lesions that form close to the brain’s fluid-filled spaces build up slowly for many years and then begin to increase much faster around the age of 60. These particular lesions are especially disruptive because they affect major communication routes in the brain. Damage deeper in the brain did not show the same strong effects.
Crucially, our findings show that brain aging depends more on how much white-matter damage a person has than on their age alone. Two people of the same age can have very different brain-aging trajectories depending on whether they have crossed this damage threshold.
Overall, our work shows that white-matter damage is not just a silent consequence of aging – it can actively speed up brain aging. Recognizing this early opens the door to better monitoring and preventive strategies aimed at protecting brain health long before noticeable cognitive problems appear.
“Because axonal pruning progresses differently in each person, brain health and aging are not the same for everyone.” – Dr. Vivek Tiwari
What are the potential implications of your findings for the field and society?
One of the most important implications of this work is the ability to distinguish normal brain aging from accelerated aging using a clear, quantitative biological threshold. In simple terms, individuals whose white-matter damage remains below a critical level tend to follow a more typical aging trajectory, whereas those who cross this threshold show signs of faster brain aging and are likely to benefit from closer monitoring and proactive management of vascular risk factors such as high blood pressure.
At a societal level, this is particularly relevant because vascular risk factors are rising worldwide. As a result, the burden of cerebrovascular injury and age-related cognitive decline is likely to grow beyond what would be expected from aging alone. Our findings highlight that brain vulnerability depends not just on age, but on the cumulative burden of small-vessel–driven white-matter injury, which varies widely between individuals.
For the field, these results challenge how “healthy aging” is currently defined. The findings argue for a shift toward quantifying white-matter lesion burden early, before overt cognitive decline, rather than treating such changes as secondary or incidental. This framework can improve how individuals are stratified in aging studies and clinical trials, sharpen vascular-based models of neurodegeneration, and support earlier, preventive interventions aimed at preserving brain health.
Overall, the work reframes white-matter injury as a modifiable driver of brain aging, with implications for public health, prevention strategies, and how societies prepare for cognitive aging in an increasingly vascular-risk–burdened world.
What was the exciting moment during your research?
One of the most striking – and frankly concerning – moments was realizing that many people who performed perfectly well on standard cognitive tests already carried a substantial burden of white-matter damage in their brains. It revealed how much silent brain injury can accumulate without obvious symptoms, long before problems become clinically apparent.
The real breakthrough came when we discovered a clear, shared threshold for this damage. Once lesions near the brain’s ventricles crossed a modest volume – approximately 2.3 mL – we consistently saw accelerated brain tissue loss, breakdown of major communication pathways, and measurable difficulties in skills like attention and word retrieval. At that point, the data made it unmistakably clear: these changes are not harmless signs of aging, but active contributors to brain vulnerability. That realization fundamentally changed how we think about brain aging.
Paper reference: Gupta, N. K., Yadav, N., & Tiwari, V. (2025). Brain aging and cognitive decline accelerate beyond a threshold of periventricular white matter hyperintensity. Cerebral Cortex, 35(11). https://doi.org/10.1093/cercor/bhaf302. https://academic.oup.com/cercor/article/35/11/bhaf302/8317409
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




