Excite-state proton coupled electron transfer (ES-PCET) process-based PDT agents: Pathway to a paradigm shift in photodynamic therapy
Research Summary: We provide a strategy to overcome oxygen dependence in photodynamic therapy using cyclometalated Ir(III) complexes to induce immunogenic cell death by excited-state proton-coupled electron transfer process with high phototherapeutic efficiency.
Author interview

Banshi Roy is pursuing PhD on design of smart metal complexes for spatiotemporal control of drug activity at Indian Institute of Science Education and Research Kolkata under the supervision of Prof. Arindam Mukherjee.
Twitter: https://x.com/BANSHIROY4
Instagram: https://instagram.com/roybanshi05
Lab: Prof. Arindam Mukherjee, Indian Institute of Science Education and Research Kolkata
Lab website: https://arindammukherjee.weebly.com/
Lab social media: https://x.com/SMTL_IISERK
What was the core problem you aimed to solve with this research?
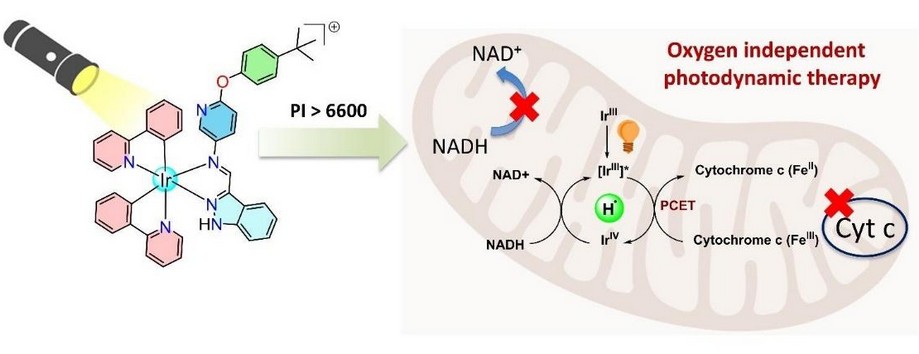
The main challenge we aimed to solve was that current photodynamic therapy (PDT) treatments need oxygen to work, making them ineffective in oxygen-poor (hypoxic) tumors which is a hallmark of solid cancers. Our research has developed light-activated Iridium (Ir(III)) compounds based on ES-PCET that would kill cancer cells even when oxygen is low. In addition, the killing pathway is immunogenic apoptotic cell death. By carefully modifying the attached organic molecules to the metal ion, called ligands, we boosted the light-triggered activity from around 150 to 6600 times compared to earlier designs, offering a powerful new strategy to increase efficiency of ES-PCET based PDT agents.
How did you go about solving this problem?
The idea originated with my supervisor, while exploring the mechanistic aspects of using biologically relevant pharmacophores such as imidazole and benzimidazole in metal complexes for therapeutic purposes. The earlier lab senior studied the control chemotherapeutic responsiveness by modulating the electronic interactions of the –NH group and found that replacing –NH with –NCH₃ suppressed chemotherapeutic activity, inspiring a new strategy to trigger hydrogen atom, rather than proton dissociation upon light activation. Our group earlier designed a BODIPY-Limantrafin based compound (doi.org/10.1002/smll.202505316) which gave the realization that Limantrafin boosted the PDT effect but could not stimulate the ES-PCET. Guided by this approach, I synthesized Iridium (III) complexes exhibiting excited-state proton-coupled electron transfer (ES-PCET) under white light using a Limantrafin–imidazole framework which became an exciting breakthrough. Further tuning with an indazole-based ligand, as proposed by my supervisor, produced a compound with dramatically enhanced ES-PCET efficiency.
We set out to overcome photodynamic therapy’s oxygen dependence, and it’s exhilarating to see our first breakthrough towards that goal. — Prof. Arindam Mukherjee
How would you explain your research outcomes (Key findings) to the non-scientific community?
Photodynamic therapy is a cancer treatment that uses special drugs activated by light to produce toxic molecules that kill cancer cells. However, this process normally depends on oxygen, which is often scarce inside solid tumors, making the treatment ineffective. Our approach removes this oxygen dependency by creating a compound that acts like a light-driven catalyst which can repeatedly release hydrogen atoms when illuminated and borrow hydrogen from tumor’s own natural molecules like NADH making the process catalytic. In oxygen-poor (hypoxic) tumors, this approach allows the compounds to stay active by transferring charged tiny particles (electrons/Hydrogen atoms) and disrupting vital cell processes. Our work marks an exciting breakthrough in increasing efficiency of light-based cancer therapies even in oxygen limited conditions.
What are the potential implications of your findings for the field and society?
At present the compound we designed mainly operates with white light and to make it clinically more relevant we need to tune it now for activation by near Infrared light of 650–950 nm to allow deeper tissue penetration for activation. This could significantly expand the scope of PDT where oxygen levels are low. Our success would potentially offer drug candidates that can expand the scope of photodynamic therapy.
What was the exciting moment during your research?
The most thrilling moment was when we observed that our indazole-Limantrafin based compound remained non-toxic to cancer cells in the dark but, upon white light exposure, it killed the cancer cells with an astonishing phototherapeutic index above 6600 and, while performing mechanistic studies the indazole-Limantrafin based compound was in fact efficiently performing ES-PCET using NADH and reducing the Iron center of the electron transport protein cytochrome C.
Paper reference: Roy, Banshi; Ghosh, Shilpendu; SenGupta, Soumee; Chanda, Shamik; Roy, Shantanu Saha; Sen, Sangita; Das, Rahul; Acharya, Moulinath; Mukherjee, Arindam. Indispensable Role of Indazole −NH in Cyclometalated Ir(III) Complexes to Drive ES-PCET with High Phototherapeutic Index. J. Am. Chem. Soc. 2025. 10.1021/jacs.5c15369
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.





Nice work Banshi vai! Keep it up
Thank you sankha