Research Summary: Structurally distinct glycated α-synuclein protein leads to earlier onset of Parkinsonian symptoms in mice due to altered pathology. This explains the increased risk of Parkinson’s disease (PD), in the diabetic population.
Author interview

Akshaya Rajan is a graduate student in the Thakur Neurodegeneration lab, IISER-TVM, supervised by Dr. Poonam Thakur. Her research focuses on understanding the link between diabetes and Parkinson’s disease.
Linkedin | Twitter | Instagram
Lab: Dr. Poonam Thakur, Indian Institute of Science, Education and Research, Thiruvananthapuram
What was the core problem you aimed to solve with this research?
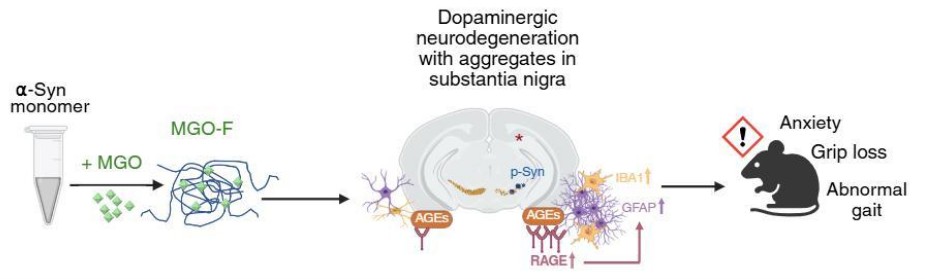
Epidemiological reports suggest that diabetes increases the risk of developing PD, but the causal molecular mechanisms linking the two remain poorly understood. Previous research has shown that a hyperglycating environment can enhance PD pathology. In this study, we specifically glycated the α-synuclein protein and investigated how its structural and pathological characteristics differ from well-established preformed fibrils of α-synuclein. We found that the resulting amorphous glycated α-synuclein assemblies led to an earlier onset of PD-like symptoms in mice, suggesting a distinct and potentially more pathogenic mechanism of disease progression.
How did you go about solving this problem?
We first studied the structural modifications induced by the glycating agent, methylglyoxal, on the α-synuclein protein aggregation. Using a range of biophysical techniques, we found that glycated α-synuclein adopts a distinct structure compared to typical fibrils or monomers.
We then designed an in-vivo experiment where glycated and non-glycated α-synuclein aggregates were injected into separate groups of mice, alongside a control group, to study PD progression and pathology. Importantly, we included both male and female mice to explore sex-specific differences, which are often overlooked in similar studies.
Throughout the study, we conducted regular motor and non-motor behavioral tests to track the disease progression. Later, histological analyses were performed to assess different PD hallmarks. Finally, we examined the Advanced Glycation End products (AGEs) and their receptor (RAGE) levels. We observed elevated levels of RAGE specifically in the substantia nigra of mice injected with glycated α-synuclein, suggesting a mechanistic link between glycation and neurodegeneration.
How would you explain your research outcomes (Key findings) to the non-scientific community?
PD is a neurodegenerative motor disorder whose global incidence is increasing. α-synuclein is a key protein whose misfolding and aggregation lead to degeneration of neurons involved in movement control. Diabetes, a major lifestyle disease, is known to increase the risk of developing PD. One reason for this may be a process called glycation, where sugar-related molecules bind to proteins and interfere with their normal function. In our research, we studied what happens when α-synuclein is glycated, a process that is elevated in diabetic individuals. We injected this modified protein into mice and found that they showed an earlier onset of PD symptoms compared to mice injected with the unmodified fibrils. We also saw more inflammation in the brains of these mice, which could be part of why their symptoms were worse. These findings suggest that glycation of α-synuclein might be one of the reasons why diabetes increases the risk of PD. In the future, if we can find ways to prevent or block glycation, we might be able to reduce the risk or severity of PD in people with diabetes.
“Diabetes can cause excessive glycation of many biomolecules, but we wanted to know why it sometimes increases Parkinson’s risk. Our study shows that when α-synuclein undergoes glycation, its structure and behaviour change in ways that trigger inflammation in the brain—helping explain the diabetes-Parkinson’s link.” – Dr Poonam Thakur
What are the potential implications of your findings for the field and society?
Our findings highlight a potential molecular link between diabetes and PD, which could have significant implications for both research and public health. This study suggests that glycation modification triggered by hyperglycemia can directly alter the dynamics of α-synuclein protein, rendering it more pathogenic. This could inspire future research into therapeutic strategies that specifically target glycation-related pathways in PD.
From a societal perspective, our work highlights the importance of managing metabolic health to reduce the risk of developing neurodegenerative conditions like PD. This is particularly relevant for India, which is rapidly becoming the diabetes capital of the world. Preventing a future rise in PD cases in such populations requires early interventions and increased awareness of the metabolic–neurological connection. In the long term, targeting glycation could lead to new treatments or preventive strategies for “at-risk” populations, potentially reducing the burden of PD on individuals, families, and healthcare systems.
What was the exciting moment during your research?
One of the most exciting moments during the research was consistently observing a distinct glycated α-synuclein structure using various biophysical techniques. This was also personally rewarding, as it provided the opportunity to learn and apply advanced analytical methods. It was more surprising that, despite lacking the typical fibrillar structure, glycated α-synuclein still led to significantly worse PD-like behaviors in mice. It highlighted just how powerful a seemingly simple sugar-related modification can be.
Additionally, while previous studies had shown increased RAGE levels in a hyperglycating environment, we were surprised to find that glycation of just a single pathogenic protein, α-synuclein was enough to elevate RAGE levels. This result pointed to a much more direct and potent link between glycation and disease progression.
Figure Caption: The amorphous glycated α-synuclein assemblies upon injection in the mouse brain lead to comparable PD-specific hallmarks with increased inflammation in the substantia nigra mediated via RAGE. This leads to earlier PD-behavioral deficits in mice. (created using Biorender, source publication- https://pubs.acs.org/doi/10.1021/acschemneuro.5c00428)
Paper reference: Rajan, et al., Glycated Alpha-Synuclein Assemblies Cause Distinct Parkinson’s Disease Pathogenesis in Mice. ACS Chem. Neurosci. (2025). https://pubs.acs.org/doi/10.1021/acschemneuro.5c00428
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




