EhASF1: The first histone chaperone uncovered from Entamoeba histolytica
Research Summary: We identified and characterized EhASF1, the first histone chaperone from Entamoeba histolytica. It binds H3/H4 dimers and promotes nucleosome assembly, revealing conserved chromatin mechanisms in this early-diverging parasite.
Author interview

Surajit Gandhi is a PhD student at BRIC–Institute of Life Sciences. He employs structural and biochemical approaches to investigate the functional mechanisms of various proteins.
Linkedin: linkedin.com/in/surajit-gandhi-32413212a
Twitter: x.com/Surajit157
Instagram: https://www.instagram.com/surajitgandhi/
Lab: Dr. Dileep Vasudevan, BRIC-RGCB Thiruvananthapuram (Previously with BRIC-ILS Bhubaneswar)
Lab social media: x.com/lab_vasu
What was the core problem you aimed to solve with this research?
Amoebiasis is a major global disease caused by Entamoeba histolytica, yet the chromatin biology of this human parasite remains poorly understood. No histone chaperone had ever been characterised in this organism, leaving a fundamental gap in understanding how it organises its highly AT-rich genome. Our goal was to uncover ASF1, a universally conserved H3/H4 histone chaperone from E. histolytica, how it has evolved, and whether it still performs its core DNA-packaging functions in this early-diverging eukaryote.
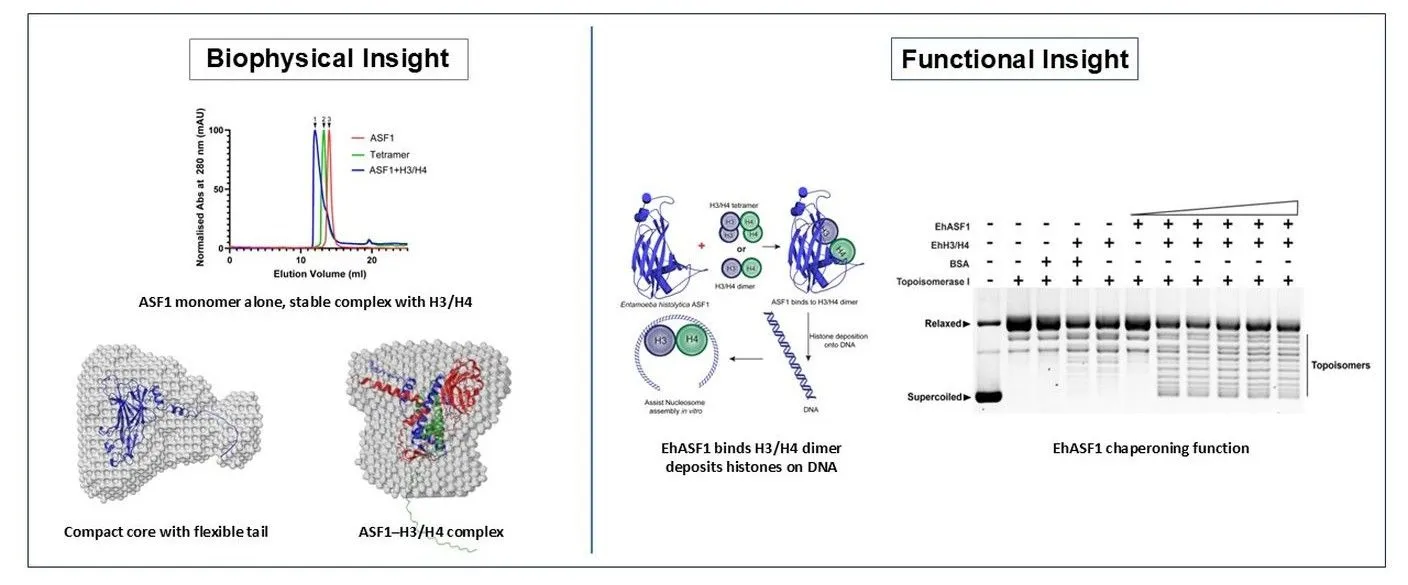
How did you go about solving this problem?
We began by identifying the ASF1 homolog bioinformatically and then cloned, expressed, and purified the recombinant protein. To understand its behaviour, we used CD spectroscopy, Analytical SEC, SV-AUC, SAXS, and ITC to define its secondary structure, oligomeric state, overall architecture, and histone-binding properties. Finally, plasmid supercoiling and EMSA-based deposition assays were used to test whether EhASF1 could deposit histones onto DNA, confirming its function as a histone chaperone.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Think of DNA as a long thread that needs to be tightly and neatly packed for a cell to store and use the information properly. This packing is done by histones, small proteins that act like spools, but within the nucleus, histones do not properly assemble on DNA on their own. They need helpers called histone chaperones. In our work, we discovered and characterised EhASF1, the first histone chaperone identified in Entamoeba histolytica. This protein binds to histones, protects them, and delivers them to DNA, allowing the parasite to assemble and maintain its genome. What’s striking is that this ancient parasite employs the same fundamental DNA packaging strategy found in higher organisms.
ASF1 is the first histone chaperone to be characterized from Entamoeba histolytica. — Dr. Dileep Vasudevan
What are the potential implications of your findings for the field and society?
Understanding how the parasite assembles and regulates its DNA helps reveal how it survives inside humans. Histone chaperones play a central role in gene expression control, making EhASF1 and related factors potential new molecular targets for anti-amoebic strategies. The work also expands the evolutionary understanding of chromatin assembly in early-diverging eukaryotes.
What was the exciting moment during your research?
Seeing relaxed plasmid DNA resolve into different topoisomeric forms only when EhASF1 was present made its role as a functional histone chaperone immediately clear. That gel image not only confirmed the function; it tied together everything our biophysical data had predicted.
Paper reference: Gandhi S, Vasudevan D. Characterisation of Entamoeba histolytica anti-silencing function 1 as a histone chaperone. Biochimie. 2025 Nov 11: S0300-9084(25)00266-4. doi: 10.1016/j.biochi.2025.11.003. PMID: 41232797.
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




