Investigation of Neural stem cell (NSC) growth and mitosis regulation in the developing central nervous system (CNS) of Drosophila
Research Summary: The Drosophila larval brain provides an excellent model for investigating NSC biology. Determining how the NSC’s fate is determined in different parts of the nervous system is an important question to study. Our study shows that the Hox gene AbdA acts as a critical regulator of NSC growth, size, duration, and mitotic rate during the developing Drosophila CNS.
Author interview

Papri Das is a Ph.D. scholar at Banaras Hindu University. Her work focuses on identifying the fate determinants of Drosophila neural stem cells that regulate their growth and mitotic potential during CNS development.
Linkedin: https://www.linkedin.com/in/papri-das-98775b2b4/
Instagram: https://www.instagram.com/papri1841
Lab: Dr. Richa Arya, Banaras Hindu University
Twitter: https://x.com/Richa__arya
What was the core problem you aimed to solve with this research?
Not all stem cells behave the same way; their potential varies depending on their spatial location and age. Since we observe a very prominent NSC size heterogeneity in the developing larval CNS of Drosophila, we ask whether NSC size has any correlation with their mitotic potential and whether there is a genetic basis. Our findings suggest that both the size of neural stem cells and their spatial context play critical roles in determining their mitotic potential, providing valuable insights into the genetic basis of stem cell behavior in the developing Drosophila CNS.
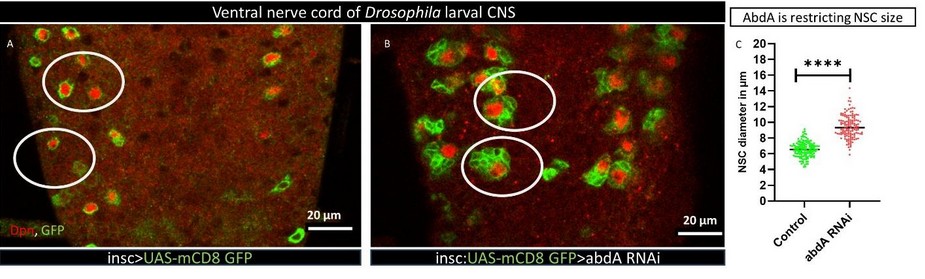
How did you go about solving this problem?
To investigate the factors that influence NSC behavior in a region-specific manner, we focused on the Hox protein abdA, a spatial transcription factor expressed only in a specific region of the CNS. Our goal was to understand its role in regulating NSC fate.
We utilized the UAS-Gal4 bipartite gene expression system to downregulate abdA expression by introducing its siRNA into NSCs. Interestingly, we found that upon knockdown of abdA, smaller NSCs grew larger in size compared to the control group as they progressed through larval life. These larger NSCs entered mitosis earlier than usual and divided at a faster rate than those in the control group.
We also noted that ectopic expression of abdA in NSCs outside its normal domain reduced their size and delayed their mitotic onset. Our mathematical analysis suggests that NSCs in the ventral nerve cord must achieve a critical size of around 6 µm to initiate mitosis. These observations indicate that abdA regulates NSC cell cycle either by influencing cell size or by directly regulating cell size and the cell cycle.
We are excited about the work. The team has worked tremendously hard on the design and execution of experiments. We are currently exploring how the size and cell cycle in NSCs are connected. — Dr. Richa Arya
How would you explain your research outcomes (Key findings) to the non-scientific community?
For the common people, I would convey a simple message that we have added new information about NSC, a cell in the brain which makes our brain by producing neurons and other brain cells, for what we accomplish our motor functions like walking, talking, eating, thinking and all. And these neurons have incredible functional diversity in the brain and they perform different motor functions. From Drosophila NSC, we have learned that NSC size regulation is important for producing the correct number of neurons in a specific region and to maintain functional harmony of CNS. In the human brain, there are also NSCs that produce different types of neurons in a specific number, dependent upon the region and the age of brain development. To have knowledge of how NSC behavior is regulated will guide us to develop better strategies for neuron-related brain disorders.
What are the potential implications of your findings for the field and society?
We found that specific genetic factors can regulate the size of NSCs, resulting in smaller cells. These smaller NSCs produce four times fewer neurons than the larger NSCs found in other regions of the CNS. Additionally, they enter mitosis later and divide more slowly. Our findings suggest that tumors caused by hyper-proliferation in stem cells could be managed by regulating stem cell size. Keeping NSCs below a critical size may help reduce tumor load.
Certainly, detailed studies are needed to understand the genetic circuitry involved in this process.
What was the exciting moment during your research?
We observed that a specific CNS region contains a population of smaller neuroblasts (NSCs). Our excitement grew when we discovered that knockdown of abdA in these small NSCs caused a pronounced increase in their size throughout larval development. AbdA, a Hox protein classically known for conferring segment-specific identity, had never been implicated in regulating NSC
Paper reference/citation (with link): Das P, Murthy S, Abbas E, White K, Arya R. The Hox Gene, abdominal-A, controls the size and timely mitotic entry of neural stem cells during CNS patterning in Drosophila. Mol Biol Cell. 2025 Nov 1;36(11):ar130. doi: 10.1091/mbc.E24-08-0347. Epub 2025 Sep 3. PMID: 40901731; PMCID: PMC12586889.
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




