Research Summary: Sulfolipid-1 from Mycobacterium tuberculosis activates TRPV4-mediated calcium influx in macrophages, promoting lysosomal biogenesis and remodeling, and revealing a previously unknown mechanism of host organelle manipulation by the pathogen.

Author interview: Ibrahim Umar is a PhD student in Dr. Varadharajan Sundaramurthy’s lab at the National Centre for Biological Sciences (NCBS), India. He investigates the cell biology of infection, with a particular focus on how Mycobacterium tuberculosis rewires host lysosomal pathways within macrophages. Outside the lab, Ibrahim enjoys exploring different cultures, history, theology, and places through YouTube, fueling his curiosity and passion for learning about the world.
Lab: Dr. Varadharajan Sundaramurthy, National Centre for Biological Sciences (NCBS)
What was the core problem you aimed to solve with this research?
Lysosomes are acidic organelles essential for immune responses, metabolism, and pathogen clearance. While classically Mycobacterium tuberculosis (Mtb) is well known for blocking phagosome–lysosome fusion through its virulence factors, our previous work revealed a striking paradox: Mtb can also enhance lysosomal content and acidification in host cells, particularly through its surface lipid Sulfolipid-1 (SL-1). However, the molecular mechanism underlying this lysosomal remodeling was previously unknown.
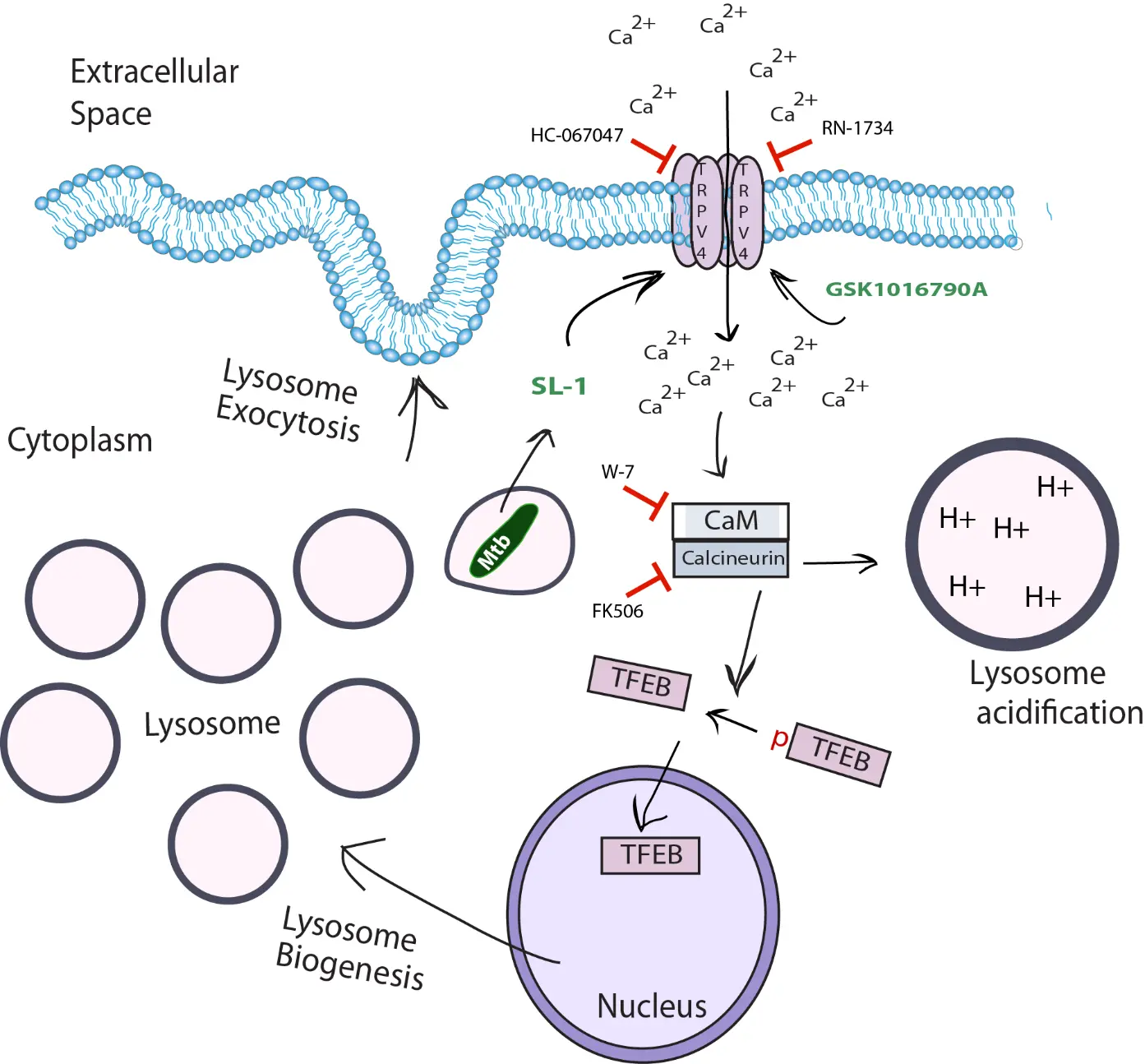
How did you go about solving this problem?
SL-1 has been shown to induce calcium influx in both neutrophils and neurons. Given that TRPV4 is a well-characterized calcium-permeable channel and has previously been implicated in regulating phagosome–lysosome fusion in macrophage during Mtb infection, we hypothesized that TRPV4-mediated calcium influx may be required for SL-1 induced lysosomal acidification and biogenesis.
We began by testing whether TRPV4 was required for the lysosomal enhancement observed upon SL-1 exposure. Using pharmacological inhibitors and genetic manipulation in macrophages, we found that blocking TRPV4 significantly impaired SL-1 induced calcium influx, lysosomal biogenesis and lysosome exocytosis, suggesting that TRPV4 is a critical mediator of this process. In contrast, chemical activation of TRPV4 alone, even in the absence of SL-1 or Mtb, was sufficient to induce robust lysosomal expansion, mimicking all the lysosomal effect of SL-1.
To explore how TRPV4-mediated calcium signals lead to lysosomal biogenesis, we dissected the downstream pathway. Our results indicate that calcium influx through TRPV4 activates the calcium-binding protein Calmodulin, which in turn activates Calcineurin, a phosphatase that dephosphorylates and activates TFEB, the master transcription factor for lysosome and autophagy genes. This leads to nuclear translocation of TFEB and transcriptional upregulation of lysosomal genes. Additionally, through high-resolution fluorescence imagining, we found that TRPV4 is not restricted to the plasma membrane, but is also present on intracellular membranes, including Mtb-containing phagosomes and lysosomes.
Together, our study combining live cell imaging, high-resolution imaging, pharmacology, and genetic tools allowed us to uncover a previously unrecognized TRPV4–Ca²⁺–Calmodulin–Calcineurin–TFEB signaling axis, exploited by a mycobacterial surface lipid, that regulates lysosomal biogenesis in macrophages.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Our immune cells have tiny built-in sensors that help them respond to their surroundings. One such sensor, called TRPV4, can detect physical and chemical changes like pressure or temperature. We found that a molecule on the surface of the Mtb, called SL-1, activates TRPV4. This activation sends signals inside the immune cell that leads to an overproduction of lysosomes, small compartments that contain enzymes to digest foreign particles or pathogens, playing a crucial role in immune defense.. However, when we blocked TRPV4, the SL-1 molecule could no longer trigger this response. This showed us that TRPV4 is a key switch the bacteria use to rewire our immune cells by modulating lysosomes.
Ibrahim uncovered the mechanism of how Mtb expands lysosomes—while revealing an unexpected, exciting new role for the well-known calcium channel TRPV4.
What are the potential implications of your findings for the field and society?
While much of the field has focused on how Mycobacterium tuberculosis (Mtb) blocks phagosome–lysosome fusion, the significance and mechanisms of the increased lysosomal content observed during infection has remained poorly understood. Our study addresses this gap by uncovering a novel role for the mechanosensitive channel TRPV4 in regulating lysosome biogenesis and homeostasis in immune cells.
These findings shift the perspective from viewing lysosomal expansion as a passive response to recognizing it as a regulated, pathogen-influenced process. Understanding this mechanism opens up exciting possibilities: TRPV4 could be a host-directed therapeutic target to modulate immune responses during Mtb infection. We next aim to understand the significance of the Mtb triggered lysosomes in Mtb pathogenesis to understand why Mtb induces lysosomes.
Single nucleotide polymorphisms (SNPs) in the TRPV4 gene are often associated with gain-of-function mutations which contribute to TRPV4-linked channelopathies, caused by altered channel activity in humans. Notably, our study shows that TRPV4 activation alone is sufficient to drive lysosomal expansion. This raises the possibility that aberrant lysosomal biogenesis may underlie, or exacerbate, the pathology of TRPV4 channelopathies. These findings broaden the significance of our work beyond infection biology.
What was the exciting moment during your research?
In science, both success and failure can be exciting when they bring clarity. One memorable moment was when we used two different TRPV4 inhibitors and saw that they blocked the lysosomal expansion caused by SL-1. Even more exciting was when we activated TRPV4, a channel normally involved in sensing pressure, heat, and chemical signals, and found that this alone was enough to trigger lysosome expansion, mimicking the effect of the mycobacterial lipid.
Paper reference: Umar I, Gulzar SE, Sundaramurthy V. M. tuberculosis surface sulfoglycolipid SL-1 activates the mechanosensitive channel TRPV4 to enhance lysosomal biogenesis and exocytosis in macrophages. Mol Biol Cell. 2025 Jun 1;36(6):ar76. doi: 10.1091/mbc.E24-12-0560. Epub 2025 Apr 30. PMID: 40305098.
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!




