Novel insights into the folding and functional energetics of the bacterial outer membrane chaperone BamA
Research Summary: Our research unveils the differential effects of outer membrane lipids on the folding and function of the highly conserved chaperone BamA, and identifies potential target sites for antibiotics against Gram-negative pathogens.
Researcher Spotlight

Dr. Anjana George recently completed her PhD from Indian Institute of Science Education and Research, Bhopal. Her PhD work focuses on understanding membrane protein folding and interactions using biophysical techniques.
Linkedin: https://in.linkedin.com/in/anjana-george-4a8750147
Researchgate: https://www.researchgate.net/profile/Anjana-George?ev=hdr_xprf
Lab: Prof. R. Mahalakshmi, Indian Institute of Science Education and Research, Bhopal
Lab website: https://home.iiserb.ac.in/~maha
What was the core problem you aimed to solve with this research?
Antimicrobial resistance especially in pathogenic bacteria pose a significant threat to global healthcare. BamA is a highly conserved protein present in the outer membrane of all Gram-negative bacteria, including pathogens. BamA is the chaperone of outer membrane proteins, and is thus an excellent antibiotic target due to its essential nature and accessibility from the environment. Though there are several inhibitors being tested, the lack of molecular detail on the intrinsic properties of BamA limits rational design of effective antibacterial agents.
How did you go about solving this problem?
We conducted in vitro folding experiments of BamA in model membranes composed of outer membrane-specific lipids. We also generated an extensive library of BamA mutants for detailed molecular characterization. We then conducted equilibrium thermodynamic experiments to measure the stability of each mutant in different lipid environments, revealing the contributions of individual residues to overall protein stability. To identify folding pathways of BamA, we performed real-time kinetics measurements using stopped-flow spectrophotometry and Φ-value analysis, which provided structural insight into transition-state structures. We also conducted all-atom molecular dynamics simulations, which supported the experimental findings and provided insights on the free-energy landscape and conformational flexibility of the protein in the presence of different lipids. The results helped us identify key regions in BamA that can be used for rational antibacterial drug design.
How would you explain your research outcomes (Key findings) to the non-scientific community?
The emergence of antibiotic-resistant bacteria is a major global issue. One method to address this challenge is to target cellular machinery that the bacteria find difficult to modify to gain resistance. BamA is an essential membrane protein that helps in the assembly of nearly all other membrane proteins on the outer envelope of Gram-negative bacteria. Our study finds that the membrane lipids in the BamA environment have differential effects on its folding and function. We also identified specific regions of BamA that can be targeted by novel antibiotics, which may help guide the development of efficient drugs against resistant bacteria.
We present a novel mechanistic insight on the folding pathways of bacterial BamA, and identify molecular regulators of its function. – Prof. R. Mahalakshmi
What are the potential implications of your findings for the field and society?
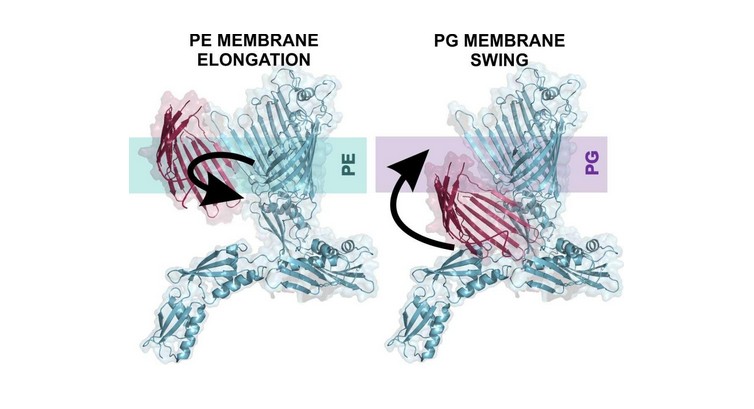
Our study demonstrates that membrane lipids actively regulate the folding pathways and conformational dynamics of the outer membrane protein BamA. We show that BamA displays unique transition state structures and folding pathways in phosphatidylethanolamine (PE)- and phosphatidylglycerol (PG)-containing membranes: PE retards folding, whereas PG promotes directional folding. Once folded, BamA is highly stable in the presence of PG-rich membranes, whereas the presence of PE induces zonal variation in stability and selective destabilization in areas that need conformational flexibility for its function. Our results indicate that chaperone–assisted molecular assembly mechanism of BamA in vivo is modulated by its lipid environment and may follow different pathways. Our study advances fundamental knowledge in membrane protein folding, and emphasizes the role of the membrane. We also identify regions in BamA sites that can serve as novel hotspots for structure-based design of peptidomimetics to target multi-drug resistant Gram-negative pathogens.
What was the exciting moment during your research?
Discovering that BamA adopts different transition-state structures in PE and PG was exciting, because it revealed distinct folding pathways for the same protein.
Paper reference: George, A., Raj, A. M., Patil, A. G., Kumari, V., & Mahalakshmi, R. (2026). Lipid-regulated assembly mechanisms and functional energetics of the essential bacterial chaperone BamA. Chemical Science. https://doi.org/10.1039/D5SC07027A
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




