Turning Reducing Sugars into Platform Chemical Using Water Microdroplets
Research Summary: Water microdroplets spontaneously convert reducing sugars into 5-hydroxymethylfurfural under ambient conditions without catalysts or heat, revealing a sustainable biomass pathway and a plausible prebiotic route linking simple sugars to heterocyclic molecules.
Researcher Spotlight
Abhijit Nandy is a PhD researcher exploring how air–water interfaces of microdroplets drive surface-confined, ultrafast, catalyst-free reactions relevant to sustainable chemistry and prebiotic chemical processes.
Linkedin: https://in.linkedin.com/in/abhijit-nandy-168a971b1
Twitter: @AbhijitNan85308
Instagram: avijit.nandy.007
Lab: Dr. Shibdas Banerjee, Indian Institute of Science Education and Research, Tirupati
Lab social media: https://www.facebook.com/BanerjeeGroup
What was the core problem you aimed to solve with this research?
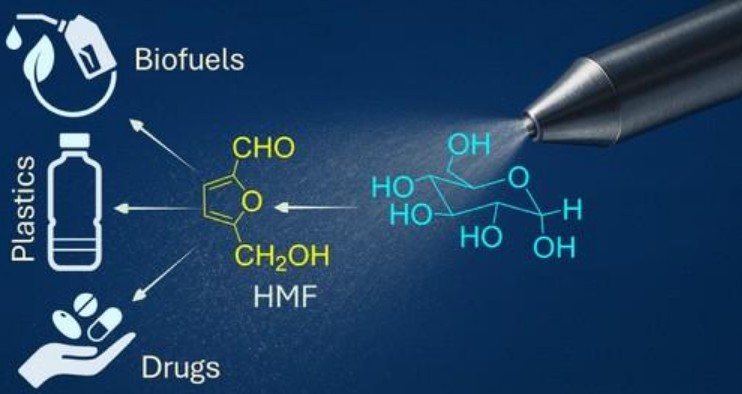
Producing 5-hydroxymethylfurfural (HMF) from sugars conventionally requires strong acids, high temperatures, and added catalysts, making the process energy-intensive and environmentally unsustainable. We tried to solve it by using a more benign solvent, water.
At the same time, carbohydrates such as glucose are among the most abundant and prebiotically plausible molecules on early Earth. Developing a greener, water-based route that converts simple sugars into furan-containing structures is therefore crucial, as furan motifs are central to nucleotides and may provide a direct chemical bridge to the origin of life. Thus, our finding is inspired by the chemical evolution, leading to the green route for the synthesis of a valuable platform chemical.
How did you go about solving this problem?
Water microdroplets act as tiny reaction vessels, a concept first demonstrated by my PI, Dr. Shibdas Banerjee, in 2011, where new chemical reactivity emerges at the gas-liquid interface that is fundamentally inaccessible in bulk phase (Journal of the American Society for Mass Spectrometry, 2011, 22, 1707-1717). Since then, numerous studies have shown that water microdroplets possess a suite of unique physicochemical characteristics, including intense electric fields at the droplet surface, partial solvation of reactants, molecular confinement, reduced surface pH, production of ROS species, and a water-deficient/dehydrating interfacial environment. Together, these interfacial features transform ordinary water into a “wonder chemical,” enabling and accelerating a wide range of chemical reactions that are otherwise slow or impossible under conventional aqueous conditions. In this study, we exploit the superacidity and water-deficient nature of the interface of water microdroplets to intrinsically activate sugars, enabling their ultrafast dehydration into HMF without the need for external catalysts or heating.
‘The surface of water microdroplets renders a dehydrating microenvironment that can fry sugar into a platform chemical 5-hydroxymethylfurfural”. — Dr. Shibdas Banerjee
How would you explain your research outcomes (Key findings) to the non-scientific community?
One can achieve a remarkable chemistry, almost like an alchemy, by the simple use of air and water, and that is nothing but a spray. If a sugar solution is sprayed into the air, the tiny droplets can make an otherwise almost impossible job for a chemist to turn sugar into a chemical under ambient and environmentally benign conditions. This chemical is commonly known as HMF (5-hydroxymethylfurfural), a valuable starting material for the production of biofuels, bioplastics, and various pharmaceuticals. This development is also industrially viable.
What are the potential implications of your findings for the field and society?
This work opens a green, energy-efficient route for converting biomass into fuels, plastics, and pharmaceuticals, while offering new insight into chemical processes that may have occurred on early Earth conditions.
What was the exciting moment during your research?
Seeing glucose, ordinarily stable in bulk water, undergo ultrafast dehydration at the surface of water microdroplets was profoundly striking. That water itself, under interfacial confinement, can act as a dehydrating agent reveals an unexpected and fascinating dimension of aqueous microdroplet chemistry for value-added chemical synthesis.
Paper reference: Water Microdroplets Transform Reducing Sugars into a Platform Chemical 5-Hydroxymethylfurfural. Abhijit Nandy and Shibdas Banerjee*. Am. Chem. Soc. 2025, 147, 51, 47810–47816 (DOI: https://doi.org/10.1021/jacs.5c18607)
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




