Research Summary: Investigated how antihypertensive drugs epigenetically modulate DNA methylation, miRNA regulation, and protein expression in endothelial cells, revealing impacts on drug response variability, adverse effects, and potential therapeutic repurposing.
Author interview
Samyukta Bhass is a researcher specializing in pharmacoepigenomics, focusing on how epigenetic changes induced by antihypertensive drugs affect gene regulation and clinical treatment outcomes.
Linkedin: www.linkedin.com/in/samyukta-bhass
Lab: Dr. Moinak Banerjee, Rajiv Gandhi Centre for Biotechnology, Trivandrum, Kerala
Lab social: https://www.facebook.com/humanmoleculargenetics.rgcb
What was the core problem you aimed to solve with this research?
Antihypertensive drugs are essential for managing hypertension, a leading modifiable risk factor for cardiovascular and cerebrovascular diseases. However, treatment effectiveness is limited by significant inter-individual variability in drug response, with only about 40% of patients achieving optimal blood pressure control. Many patients experience adverse drug reactions, leading to poor adherence and treatment discontinuation. Despite available therapies, only 21% of treated individuals benefit fully, partly due to the “trial and error” approach in prescribing these medications.
Underlying this variability are molecular mechanisms including genetic polymorphisms and emerging epigenetic factors like DNA methylation and microRNA (miRNA) regulation, which influence gene expression relevant to drug response. While pharmacogenetics explains some variability, the impact of antihypertensive drugs themselves on epigenetic regulation, especially miRNA and DNA methylation in vascular endothelial cells, remains largely unexplored.
This study seeks to fill this gap by investigating how commonly prescribed antihypertensive drugs modulate miRNA expression, DNA methylation, and downstream protein changes in human aortic endothelial cells. Understanding these pharmacoepigenetic effects aims to inform safer, more personalized therapies for hypertension, improving efficacy and minimizing side effects.
How did you go about solving this problem?
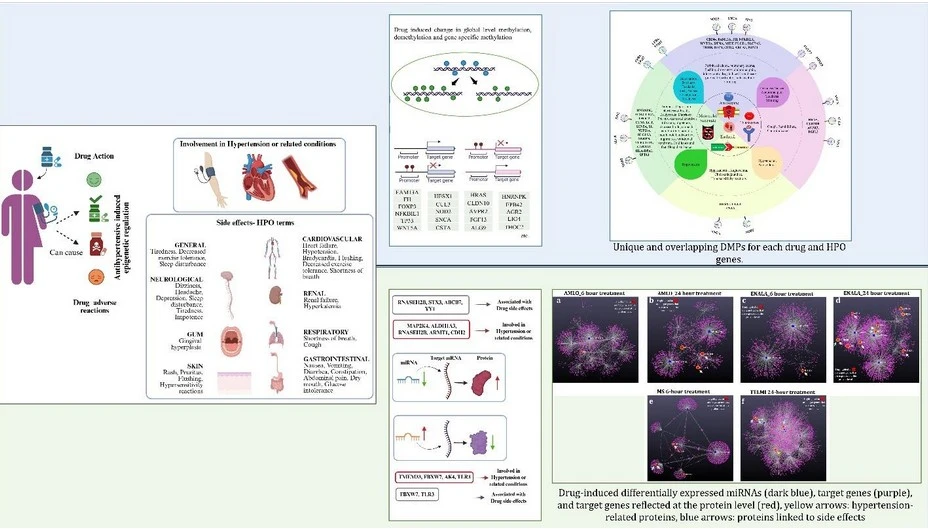
We approached this problem through a comprehensive in vitro experimental framework using human aortic endothelial cells (HAECs) to investigate the epigenetic modulations induced by antihypertensive drugs. Cells were cultured under controlled conditions and treated with clinically relevant concentrations of representative drugs from major antihypertensive classes—Amlodipine (CCB), Telmisartan (ARB), Enalapril (ACEi), and Metoprolol (BB). RNA and DNA were extracted from treated and control cells for downstream molecular analyses.
Global and gene-specific DNA methylation levels were quantified using ELISA-based kits and the Illumina Methylation EPIC 935K array to identify differential methylation patterns. Small RNA sequencing was performed on high-quality RNA samples to profile the miRNome changes induced by drug treatments. We integrated epigenetic data with proteomic analyses from LC-MS/MS of protein extracts to elucidate post-transcriptional regulatory networks.
For multi-omics integration, we analyzed differentially methylated genes (DMGs) that are linked to hypertension, related conditions, or drug side effects by referencing publicly available databases such as GWAS and the Human Phenotype Ontology (HPO) Additionally, we used bioinformatics tools such as miRWalk to identify validated miRNA target genes and miRNet for network analysis of miRNA-target gene-protein interactions. Overlapping genes and proteins that showed inverse relationships with miRNA expression changes were prioritized to elucidate the molecular mechanisms underlying therapeutic responses and adverse effects. This integrative analysis enabled a comprehensive understanding of how antihypertensive drugs epigenetically regulate gene and protein networks involved in treatment efficacy and safety.
This multi-omics, integrative approach allowed us to comprehensively characterize how antihypertensive drugs epigenetically reprogram hypertension/ side effects associated genes and protein expression, providing crucial insights into drug response variability and paving the way for personalized hypertension therapies.
The study highlights the significance of pharmacoepigenetics in precision medicine of antihypertensive drugs. This integrated OMICs based approach provides critical biomarkers that can aid in resolving adverse events associated with antihypertensives. In future individuals can develop AI tools to benefit from alternate mechanisms to reverse these adverse events or comorbidities associated risks, possibly through nutritional choices, yoga etc. The outcome of this study has tremendous potential and indeed credit goes to Samyukta for this wonderful piece of work which will further motivate her to continue on translational work. — Moinak Banerjee
How would you explain your research outcomes (Key findings) to the non-scientific community?
Our study revealed that common blood pressure medicines influence tiny chemical tags on our DNA, known as DNA methylation, along with small molecules called microRNAs, which regulate gene activity by turning genes on or off. These drug-induced epigenetic changes help explain why some individuals respond well to antihypertensive treatments while others experience side effects or limited benefits. By understanding these reversible molecular modifications, we can better tailor treatments to restore a healthy balance, improving effectiveness and reducing adverse reactions. This, in turn, promotes better adherence to therapy, ultimately enhancing patient outcomes and quality of life. Our findings underscore the importance of considering epigenetic mechanisms in personalized hypertension management to make treatments safer and more effective for everyone.
What are the potential implications of your findings for the field and society?
Our findings highlight that understanding drug-induced epigenetic changes can transform personalized hypertension treatment. By identifying how antihypertensive drugs influence DNA methylation and microRNA networks, we can better predict individual patient responses and potential side effects, moving away from conventional trial-and-error prescriptions. This knowledge enables clinicians to tailor therapies based on a patient’s molecular profile, improving treatment effectiveness and minimizing adverse reactions. Moreover, the discovery of drug-specific gene methylation, miRNA regulation and protein regulation patterns opens avenues for repurposing these drugs in treating other conditions. Collectively, our study advances precision medicine in hypertension by offering a molecular roadmap for safer, more efficient therapies, ultimately benefiting both clinical practice and patient quality of life.
What was the exciting moment during your research?
Given that antihypertensives are used by many individuals around us, in our homes and neighborhoods, with some people experiencing beneficial effects while others suffer from side effects, there remains surprisingly little concern about this variability in our daily lives. This gap inspired us to examine how antihypertensive drugs influence epigenetic mechanisms, which are both reversible and inducible, to understand how these changes may support treatment or contribute to adverse effects, and whether addressing them could help people starting from our households to populations worldwide. Thus, the research journey itself was fascinating from the very beginning, with each step unravelling new insights. However, the most thrilling and rewarding moment was discovering specific genes and proteins whose epigenetic regulation by antihypertensive drugs closely aligned with known therapeutic outcomes and adverse side effects. This finding provided a clear molecular basis for the variability in drug responses observed clinically, offering profound insights into why some patients benefit while others experience harmful effects. Additionally, uncovering the novel potential for repurposing these drugs to treat a range of other diseases opened exciting new avenues for future research and therapeutic innovation. This dual revelation of linking epigenetic mechanisms to both drug efficacy and potential new uses made the effort deeply satisfying, underscoring the impact that epigenetics can have on personalized medicine and the broader field of pharmacology.
Paper reference:
- Samyukta Bhass and Moinak Banerjee (2025). Pharmacoepigenomic Impact of Antihypertensive Drugs on miRNome and Proteome and Its Potential Influence on Health and Side Effects. Cells, 14(17), 1359. https://doi.org/10.3390/cells14171359
- Samyukta Bhass and Moinak Banerjee (2025). Pharmacoepigenomic effects of anti-hypertensive drugs on DNA methylation and its implication to drug response and side effects. Epigenomics, 1–16. https://doi.org/10.1080/17501911.2025.2567232
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




