The neuropathy-linked protein TECPR2: protecting cells through efficient recycling
Research Summary: This study identifies TECPR2 as a key protein that facilitates the recycling of surface receptors, preventing them from lysosomal degradation to maintain optimal cellular communication and homeostasis.
Researcher spotlight

Sankalita Paul completed her M.Sc. from the National Institute of Technology (NIT), Rourkela in Life Sciences and currently is a Ph.D. scholar working with Prof. Mahak Sharma at the Indian Institute of Science Education and Research (IISER), Mohali. Her Ph.D. research work demonstrates how TECPR2 regulates selective endosomal recycling by linking Rab5-marked cargo to recycling machinery, having implications for HSAN pathogenesis.
Linkedin : https://www.linkedin.com/in/sankalita-paul-41895732a/
Twitter: https://x.com/SankalitaPaul
Lab: Prof. Mahak Sharma, Indian Institute of Science Education and Research (IISER), Mohali
Lab website: https://web.iisermohali.ac.in:8080/faculty/mahak-sharma
What was the core problem you aimed to solve with this research?
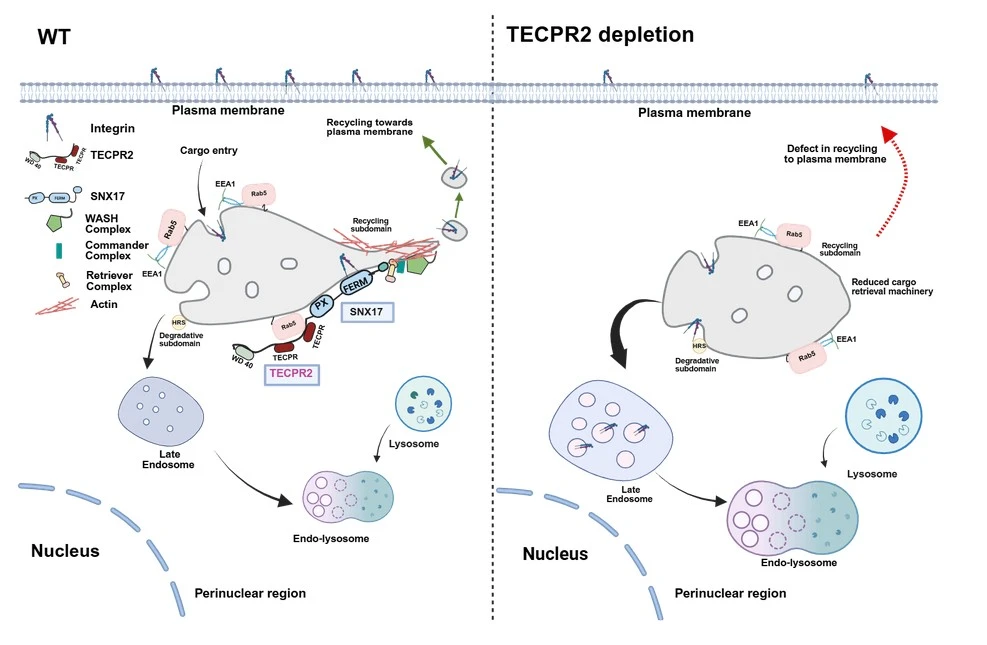
To maintain homeostasis, after internalization of cargoes, the cells constantly recycle transmembrane proteins and receptors back to the cell surface. The endosomal cargo sorting machinery accurately distinguishes whether the endocytosed proteins should be recycled back to the cell surface or directed for lysosomal degradation. Loss-of-function mutation in TECPR2 has been reported to cause a progressive neurodegenerative disorder termed Hereditary Sensory and Autonomic Neuropathy type 9 (HSAN9). We found that TECPR2 mediates cell adhesion and cell spreading and also regulates the recycling of essential cargoes like integrins by preventing mistargeting of the cargoes towards lysosomes for degradation.
How did you go about solving this problem?
In this study, we combined cell biology, advanced imaging, biochemistry, and in vivo study to uncover how dysfunction of TECPR2 disrupts cellular function relevant to HSAN9, a rare neurological disorder. Since the subcellular localization of TECPR2 was previously unknown, our first step was to determine how TECPR2 is recruited on the endosomal membrane. Using biochemical assays and immunofluorescence techniques, we discovered TECPR2 as an effector of early endosomal GTPase, Rab5, and localizes on early endosomes. Once we knew the subcellular localization of TECPR2, we investigated its functional role on early endosomes. Through GST-pulldown assays we found that TECPR2 directly interacts with SNX17, a cargo adaptor that recognizes specific motifs on integrin and facilitates its recycling to the cell surface by preventing it from lysosomal degradation. To determine the fate of integrin recycling upon loss of TECPR2, we performed an integrin recycling assay, which revealed reduced recycling of integrins to the plasma membrane and increased lysosomal degradation. Advanced live cell imaging and biochemical pulldown assays also revealed that TECPR2 regulates the recruitment of the early endosomal sorting machinery for efficient sorting of cargoes back to the cell surface. Finally, we translated these findings using zebrafish as a model organism, where tecpr2 morphants displayed motility defects and altered morphology of neuromuscular junctions. Taken together, we were able to connect the molecular interaction of TECPR2 with receptor recycling, thereby providing a mechanistic explanation of cellular defects underlying HSAN9 disorder.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Imagine cells as a factory where recyclable parts are identified and sent to sorting rooms for reuse. TECPR2 works like a quality control manager that makes sure the useable parts (cargoes like integrin, ATP7A, and EGFR) go into the recycling bin instead of the trash (the lysosomes). In the absence of TECPR2, the reusable proteins are trashed in lysosomes, disrupting the factory’s operation.
“This study has identified the subcellular role of neuropathy-linked protein, TECPR2, in mediating cargo retrieval from early endosomes to the plasma membrane.” — Prof. Mahak Sharma
What are the potential implications of your findings for the field and society?
Our findings reshape the understanding of TECPR2-associated HSAN disorder by demonstrating that the role of TECPR2 extends beyond autophagy to encompass endosomal cargo recycling as well. By serving as a vital bridge between the incoming cargo marked by Rab5-positive early endosomes and the recycling machinery, TECPR2 ensures that the key receptors are efficiently recycled rather than degraded. Disruption of this pathway explains the molecular mechanism of how TECPR2 mutations cause progressive neuronal dysfunction in HSAN9 patients. Additionally, our zebrafish results replicate the disease relevant defects found in HSAN patients and provide a platform for small-scale drug screening. Thereby, our study helps to create new opportunities to investigate strategies for treatment that address rare neurological disorders such as HSAN9-associated trafficking defects.
What was the exciting moment during your research?
In science, there is a well-known saying: “Seeing is believing”. For me, the most exciting moment of our research was the first time I looked through the microscope and visualized the subcellular localization of TECPR2. Previously, TECPR2 was thought to be mostly cytosolic; however, upon overexpression of the early endosomal small GTPase Rab5, it was recruited to these Rab5-positive compartments, indicating that it plays a critical functional role on early endosomes. Our enthusiasm increased when we translated our findings into our zebrafish model. Using whole-mount immunostaining, we verified that the wild-type protein precisely localized to the myotomes of the embryo; in contrast, the Rab5-defective TECPR2 protein exhibited diffuse expression throughout the cytoplasm. Observing this loss of localization in zebrafish provided clarity that the Rab5-defective tecpr2 mutant prevents the protein from reaching the myotomes, thereby linking mislocalization of TECPR2 to loss of Rab5 membrane localization and its disease relevance.
Paper reference: Paul, S., Pant, R., Sharma, P., Walia, K., Gupta, S., Aseem, A., … & Sharma, M. (2025). The neuropathy-linked protein TECPR2 is a Rab5 effector that regulates cargo recycling from early endosomes. Nature Communications, 16(1), 10537. https://doi.org/10.1038/s41467-025-65568-4
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




