Research Summary: Our study explores how dopaminergic neuron specification occurs in olfactory bulb and midbrain.
Author interview

Smitha Bhaskar is a trained cell biologist, who has a Master’s degree in Biotechnology. During her PhD with Dr. Anujith Kumar, she focused on understanding the role of ZIC3 in early neural development using in vivo and embryonic stem cell models.
Lab: Dr. Anujith Kumar, Manipal academy of higher education
What was the core problem you aimed to solve with this research?
Dopaminergic neurons are a set of clinically relevant neurons found in various regions of the brain. They are involved in controlling motor activity and olfactory abilities. These neurons are the major targets in Parkinson’s disease (PD): a leading neurodegenerative disorder. While the dopaminergic neurons in the midbrain undergo degeneration in PD, they witness a dramatic increase in the olfactory bulb. However, both these populations of dopaminergic neurons lose their function in the disease context and lead to loss of motor control preceded by an evident loss of olfaction.
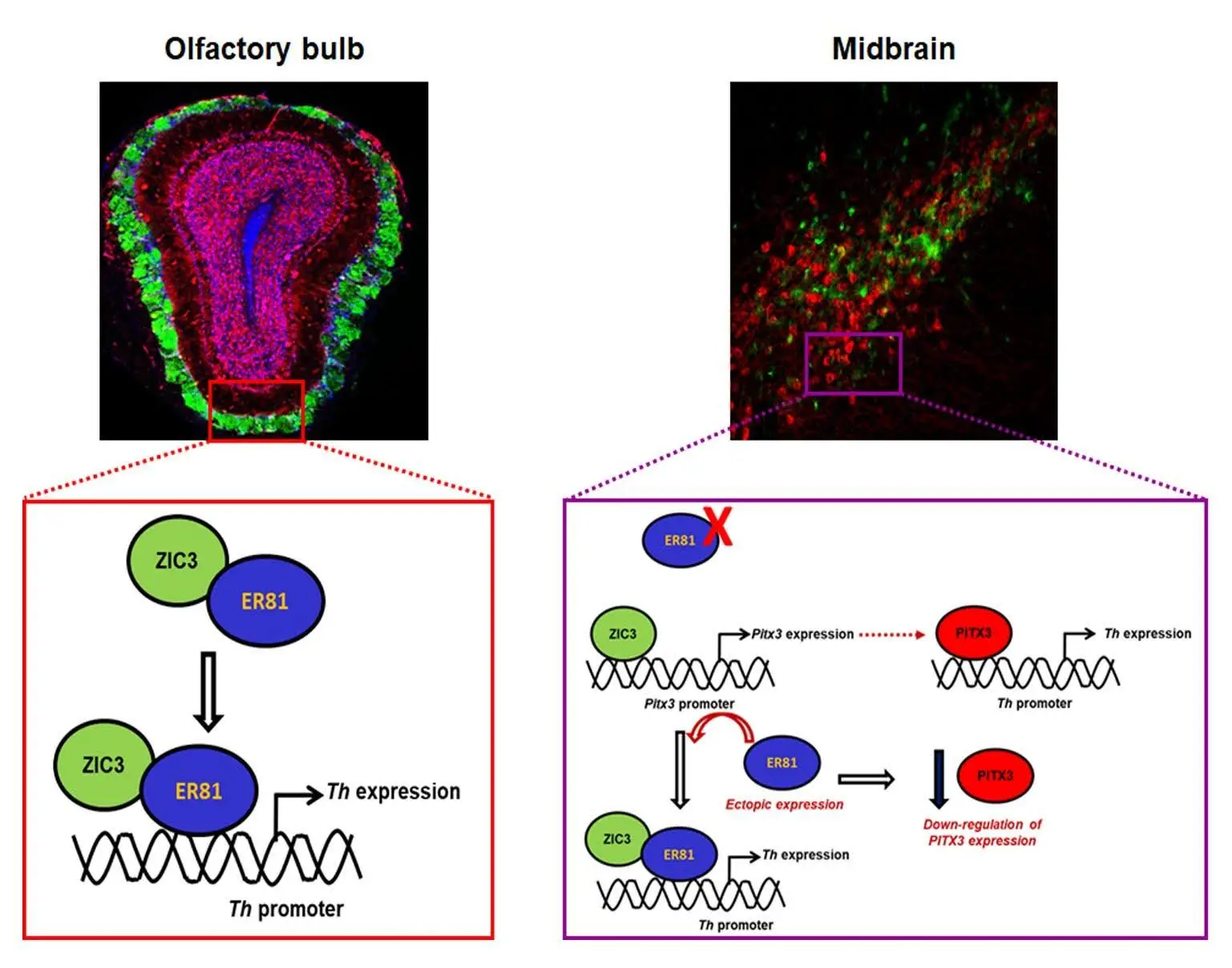
Molecular mechanisms that bring about this distinct response of midbrain and olfactory bulb dopaminergic neurons to PD insults remains poorly understood. In our study, we studied how ZIC3- a zinc finger transcription factor contributes to the bimodal regulation of these neurons in distinct brain areas. Using tyrosine hydroxylase (TH) expression as a proxy for dopaminergic neuron identity, we show that ZIC3 is a positive regulator of TH. Interestingly, ZIC3 controls the expression of TH in both these regions using different molecular mechanisms. Given the absence of any direct binding site on TH, ZIC3 interacts with a ETS family transcription factor ER81 in the olfactory bulb and facilitates its binding to the TH promoter. On the other hand, in the midbrain, where the cells lack ER81 expression, ZIC3 bypasses the protein interaction and binds directly to the promoter region of a transcription factor PITX3 which can then bind TH and drive its function.
Our in vivo data reveals a significant reduction in the dopaminergic neurons of both these brain regions at birth in the Zic3 null mice. This provides a new perspective to the way the scientific community has been viewing ZIC3. Conventionally, ZIC3 is implicated in regulation of pluripotency and body axis symmetry. Our study advocates ZIC3 to influence the dopaminergic neuron differentiation and this opens up some interesting avenues to explore the involvement of ZIC3 in PD disease initiation and propagation.
How did you go about solving this problem?
To begin with, we exposed the mice to known dopaminergic neuron inducing odor and found specific upregulation in zic3 expression, but not the other ZIC family members. Further analysis of the dopaminergic neuron population in the olfactory bulb and midbrain of ZIC3 knock out neonatal mice showed severe loss of dopaminergic neurons. In order to develop a robust model system where we can study the molecular mechanism, we utilized mouse embryonic stem cells differentiated to dopaminergic-like neurons and primary neurons obtained from embryonic mouse midbrain and the olfactory bulb. shRNA mediated knockdown of Zic3 in these experimental sets showed a significant down-regulation of TH expression thus suggesting a compromised dopaminergic-like identity. We then explored the plausible molecular mechanisms and established a protein interaction between ZIC3 and ER81 in the olfactory bulb and ZIC3 mediated PITX3 regulation in the midbrain. Both these distinct molecular associations ultimately resulted in the positive regulation of TH expression thus demonstrating ZIC3 as one of the dopaminergic neuron fate determinants.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Parkinson’s disease is one of the leading neurodegenerative disorders across the globe. Being a late onset disease, it poses formidable challenges to early diagnosis and treatment. Any fundamental knowledge of the protein involved in causing the disease would be a leap in our understanding of the disease. Our study finds a protein ZIC3 to be involved in the generation and maintenance of dopaminergic neurons that are primarily affected in Parkinson’s disease. ZIC3 affects the expression of tyrosine hydroxylase- a hallmark gene in the dopaminergic neurons in both midbrain and the olfactory bulb. This suggests that ZIC3 might be crucial for the dopaminergic neurons in these two regions of the brain and it can be one of the targets for potential therapy in the future in order to prevent the degeneration of dopaminergic neurons in PD.
What are the potential implications of your findings for the field and society?
As ours is a fundamental study, we are fairly self-aware that this might not have any direct societal applications at the moment. However, given the complexity of a neurodegenerative disorder such as Parkinson’s disease, any tiny step towards understanding the molecular mechanism that governs the generation, survival and maintenance of the dopaminergic neurons could be instrumental. In this regard, our study for the first time positions a single transcription factor upstream of TH both in the midbrain and olfactory bulb. This could be a first, small but important step towards understanding how these neurons are differentially regulated in different brain regions and how they are selectively targeted for degeneration in the midbrain while they flourish in the olfactory bulb. The reason for loss of smell that sets in a decade before PD manifestation remains an enigma till today and finding the differences in the molecular signatures of the neurons in the olfactory bulb in comparison to the midbrain seems to be the first logical approach to answer this question. We think that our study sheds light on this important aspect and shows that the same transcription could adopt different molecular mechanisms to govern dopaminergic neurons in these two regions and discovering more molecules like this could help us build a complete molecular map of the dopaminergic neurons in different regions of the brain which could then be exploited to discover suitable drug targets to treat PD.
This study highlights the importance of ZIC3 in dopaminergic neuron specification, the knowledge which could be utilized to derive dopaminergic neurons from stem cells for therapeutic purposes.
What was the exciting moment during your research?
Our initial findings suggested a specific molecular code in the olfactory bulb and the midbrain. While ZIC3 and ER81 formed an interaction in the olfactory bulb to drive TH expression in the olfactory bulb, ZIC3 had to resort to driving PITX3 in the midbrain to indirectly regulate TH in the midbrain. One of the challenging and equally rewarding experimental finding was when we over-expressed ER81 in the midbrain primary neurons and saw that ZIC3 could switch its molecular partner, displace from PITX3 promoter and interact with ER81 in a forbidden cellular type to ultimately result in positive regulation of TH. This told us that ZIC3 could flexibly modulate its molecular interactions to ensure TH expression in the differentiating dopaminergic neurons.
Paper reference: Bhaskar, S., Gowda, J., Hegde, A. et al. Zic3 enables bimodal regulation of tyrosine hydroxylase expression in olfactory bulb and midbrain-derived neurons. Cell Death Discov. 11, 165 (2025). https://doi.org/10.1038/s41420-025-02448-2
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!




