🩸 A Major Step Forward in Multiple Myeloma Care
Date: October 2025
Source: GSK Press Release
In a significant advancement for blood cancer treatment, the U.S. Food and Drug Administration (FDA) has approved GSK’s Blenrep (belantamab mafodotin-blmf) in combination with bortezomib and dexamethasone (BVd) for adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMID).
This marks an important milestone in GSK’s DREAMM clinical program, addressing one of the most persistent challenges in myeloma treatment — disease relapse and resistance to existing therapies.
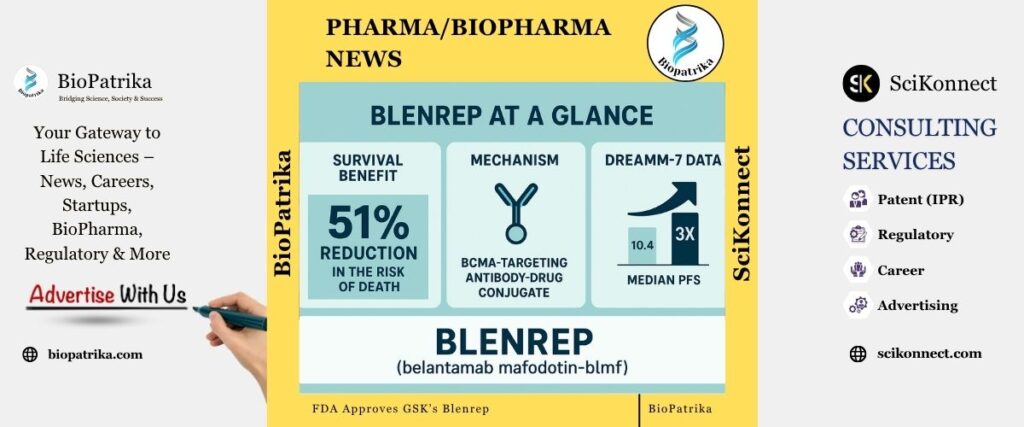
🔬 Key Highlights from DREAMM-7 Phase III Trial
The FDA approval is supported by results from the pivotal DREAMM-7 Phase III study, which compared Blenrep (BVd) with a daratumumab-based triplet (DVd) in patients with two or more prior lines of therapy.
Clinical outcomes:
- 51% reduction in the risk of death (HR 0.49; 95% CI: 0.32–0.76)
- Tripled median progression-free survival (PFS): 31.3 months vs. 10.4 months
- Safety: Tolerability consistent with the known profiles of individual agents
These findings were presented at the ASCO Plenary Series (Feb 2024) and published in the New England Journal of Medicine, underscoring the therapy’s potential to transform outcomes for heavily pretreated myeloma patients.
🧬 Addressing a Significant Unmet Need
Tony Wood, Chief Scientific Officer at GSK, highlighted the importance of this milestone:
“Today’s FDA approval of Blenrep is another significant milestone, providing potential for superior efficacy and overall survival to US patients. Nearly all patients with multiple myeloma experience relapse, and re-treating with the same mechanism of action often leads to suboptimal outcomes.”
As the only anti-BCMA therapy accessible in community settings—where around 70% of U.S. patients receive care—Blenrep bridges a critical accessibility gap. The therapy will be available under a streamlined REMS program, designed to simplify administration and monitoring while ensuring patient safety.
⚙️ A Simplified REMS and Patient Support Program
GSK, in collaboration with the FDA, has launched a new streamlined Risk Evaluation and Mitigation Strategy (REMS) for Blenrep.
This simplified program:
- Removes duplicative checklists
- Reduces administrative burden for clinicians
- Streamlines communication between treating physicians and eye-care specialists
GSK also offers “Together with GSK”, a dedicated patient support initiative providing education, financial assistance, and adherence resources for those prescribed Blenrep.
🌍 Expanding Global Access and Clinical Development
Blenrep combinations are already approved for 2L+ relapsed or refractory multiple myeloma in:
- European Union
- United Kingdom
- Japan
- Canada
- Switzerland
- Brazil
Regulatory reviews are underway in additional regions, including China, where Blenrep has Breakthrough Therapy Designation and Priority Review status.
Meanwhile, GSK continues to advance the DREAMM program:
- DREAMM-8 and DREAMM-10 are evaluating Blenrep in earlier-line and newly diagnosed settings.
- DREAMM-10 focuses on transplant-ineligible patients (70% of new diagnoses).
- Overall survival (OS) data from ongoing trials are expected in early 2028.
🧫 About Blenrep
Blenrep (belantamab mafodotin-blmf) is an antibody-drug conjugate (ADC) targeting B-cell maturation antigen (BCMA), a validated target in multiple myeloma.
It comprises:
- A humanized anti-BCMA antibody
- Linked to auristatin F, a cytotoxic agent
- Via a non-cleavable linker technology (licensed from Seagen Inc.)
The monoclonal antibody component uses POTELLIGENT® technology from BioWa Inc. (Kyowa Kirin Group) to enhance antibody-dependent cellular cytotoxicity (ADCC).
🧭 What This Approval Means for Patients
Multiple myeloma, the third most common blood cancer globally, affects approximately 180,000 new patients annually. Despite treatment advances, relapse remains nearly universal, and effective options for refractory disease are limited.
Blenrep’s approval offers:
- Improved survival outcomes
- Community accessibility
- Novel anti-BCMA mechanism
- Potential use in earlier disease lines
This development represents a paradigm shift in myeloma therapy, expanding patient access to cutting-edge antibody-drug conjugate technology.
💬 Expert Perspectives
Dr. Sagar Lonial, Chief Medical Officer, Winship Cancer Institute, Emory University:
“With Blenrep, we now have a community-accessible BCMA-targeting agent that can improve outcomes for patients following two or more prior lines of treatment.”
Michael Andreini, CEO, Multiple Myeloma Research Foundation:
“For patients facing the relentless cycle of remission and relapse, Blenrep offers new hope for longer, better-quality lives.”
❓ FAQs
1. What is Blenrep?
Blenrep (belantamab mafodotin-blmf) is an antibody-drug conjugate targeting BCMA, approved for patients with relapsed or refractory multiple myeloma after two or more prior therapies.
2. What does the FDA approval cover?
Blenrep is approved in combination with bortezomib and dexamethasone (BVd) for adults with RRMM who have received at least two prior lines of therapy, including a PI and an IMID.
3. What are the key findings from DREAMM-7?
Blenrep combination therapy showed a 51% reduction in risk of death and tripled progression-free survival compared to a daratumumab-based triplet regimen.
4. How is Blenrep administered?
It is given intravenously every three weeks, with dosing adjusted per cycle and monitored under a streamlined REMS program.
5. What’s next for Blenrep’s development?
GSK’s ongoing DREAMM-8 and DREAMM-10 trials will explore its use in earlier-line and newly diagnosed patients, with long-term data expected by 2028
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




