Malaria continues to be one of the deadliest infectious diseases worldwide, claiming nearly 600,000 lives in 2023, mostly among children in Africa. While vaccines and antimalarial drugs have advanced in recent years, researchers are still searching for stronger, longer-lasting interventions. Two new studies now offer promising directions—by revealing novel classes of antibodies that target Plasmodium falciparum sporozoites, the earliest stage of the parasite transmitted by mosquitoes.
NIH identifies hidden “Achilles’ heel” of malaria parasite
In a landmark study published in Science, researchers at the U.S. National Institutes of Health (NIH) discovered a new class of monoclonal antibodies (mAbs) that bind to a previously unrecognized site on the parasite’s circumsporozoite protein (PfCSP). Unlike existing vaccines and most tested antibodies, which target the well-studied central repeat region of PfCSP, these newly identified antibodies zero in on a cryptic epitope called pGlu-CSP. This site only becomes exposed after a natural modification step during parasite development, but once revealed, it is conserved across parasite strains.
The most potent antibody, MAD21-101, provided full protection against infection in a humanized mouse model. Crucially, because pGlu-CSP is not part of current malaria vaccines (RTS,S and R21), these antibodies could be used alongside existing vaccines without interference—potentially protecting infants or high-risk groups before vaccination coverage sets in.
“This is the first time we’ve seen protective antibodies that target outside the traditional repeat region,” said NIH scientists. “It opens the door to designing vaccines and therapies that exploit this hidden vulnerability of the parasite.”
Phage display technology yields potent human antibodies
In a complementary approach, a separate study by researchers from National Institute of Immunology and ICGEB India published in Vaccine reported the discovery of fully human antibodies against PfCSP using a phage display strategy. Screening human antibody libraries, researchers identified two unique clones—PfCSP-CL1 and PfCSP-CL3—that showed strong binding to PfCSP and inhibited sporozoite invasion of liver cells in vitro.
When tested in mice, low doses of these antibodies conferred protection comparable to that of the gold-standard antimalarial antibody 2A10. Structural modeling revealed that CL1 and CL3 bind regions of PfCSP downstream of the repeat domain, again pointing to the potential of moving beyond the classical vaccine targets.
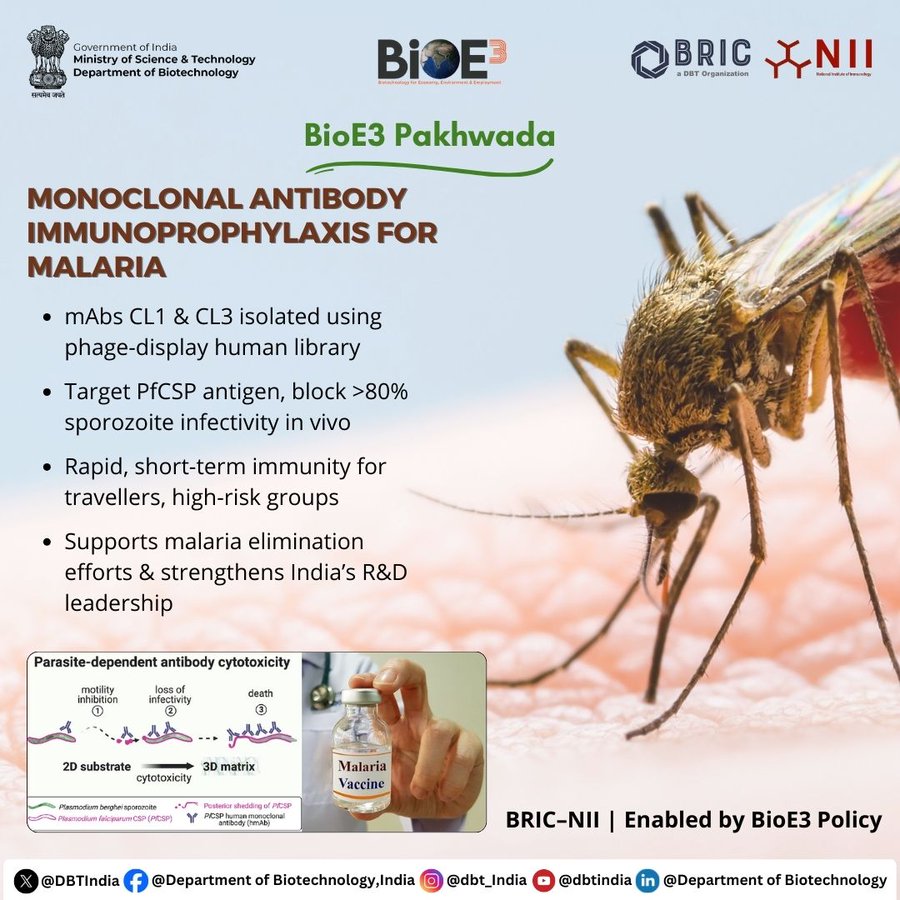
These antibodies could be developed as antibody-based prophylactics—providing rapid protection for travelers, military personnel, or during malaria elimination campaigns.
Toward a new era of malaria immunotherapy
Together, these studies highlight how innovative discovery platforms—antigen-agnostic screening and phage display—are reshaping the landscape of malaria prevention. Both efforts converge on the idea that PfCSP harbors underexplored sites of vulnerability that can be harnessed to design next-generation interventions.
While further clinical studies are needed, these findings raise hope for more effective vaccines and antibody-based therapies that could complement existing tools and accelerate progress toward malaria elimination.
🔗 Read the original studies:
- Science: Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein
- Vaccine: Novel, fully human, anti-PfCSP antibodies with potent antimalarial activity using a phage display based strategy
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




