Research Summary: We developed GD-07, a drug-like biguanidine that selectively binds the c-MYC promoter G-quadruplex, suppressing MYC-driven glycolysis and triggering apoptosis, with strong activity in ovarian cancer cells and patient-derived organoids.
Researcher Spotlight

Mamta Singh is a PhD researcher in Amity Institute of Molecular Medicine & Stem Cell Research (Amity University) focused on DNA secondary-structure targeting and cancer metabolism, integrating computation, biophysics, and organoid models to advance translational anticancer drug discovery.
LinkedIn: https://www.linkedin.com/in/mamta-singh-b42759126
Research Gate: https://www.researchgate.net/profile/Mamta-Singh-9
Instagram: https://www.instagram.com/mamtasingh4184
Lab: Dr. Vinit Kumar, Amity University Uttar Pradesh and Gautam Buddha University Uttar Pradesh
What was the core problem you aimed to solve with this research?
MYC is a major cancer driver but is notoriously difficult to inhibit directly with small molecules because it lacks a clear drug-binding pocket, leaving a big therapeutic gap especially in ovarian cancer where MYC-driven metabolic rewiring supports aggressive disease.
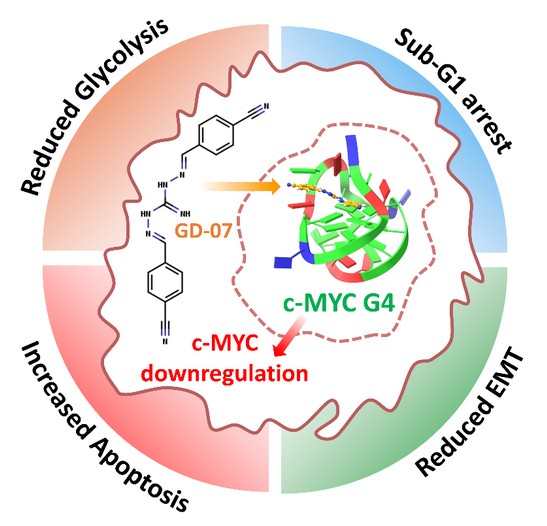
How did you go about solving this problem?
We targeted MYC indirectly by stabilizing the G-quadruplex (G4) DNA structure in the c-MYC promoter rather than trying to drug the MYC protein itself. This G4 structure is a regulatory “switch” upstream of the MYC gene. This approach helps overcome a critical barrier in the field of drug-design: targeting MYC and similar transcription factors directly is difficult because they lack a well-defined small-molecule binding site and have short half-lives. The MYC promoter contains a GC-rich region called Pu27 that can fold into a stable intramolecular G-quadruplex. Stabilizing Pu27 suppresses c-MYC expression, offering a tractable way to downregulate MYC at the transcriptional level. Using an in-silico workflow, we enumerated ~60,000 nature-inspired biguanidine analogs, applied ADME drug-likeness filters, docked candidates to the c-MYC G4, synthesized the top series, and then validated activity using biophysical (ITC/CD/NMR/MD) and biological assays (cell viability, migration, apoptosis, transcriptomics, and patient-derived organoids).
How would you explain your research outcomes (Key findings) to the non-scientific community?
Cancer cells are rapidly dividing and show abnormally high levels of metabolism. These characteristics demand regular and high-degree of energy consumption. The cancer cell facilitates this by rewiring its metabolism to utilize glucose as its primary fuel source. The MYC protein is one of the key drivers of this metabolic rewiring but targeting MYC directly has proven to be difficult due to the lack of any binding pockets on its surface. We developed a small molecule (GD-07) which indirectly reduces MYC production by targeting and stabilizing a structure called G4-qudruplex, present on the MYC gene’s promoter region. Destabilization of this G4-structure is necessary for transcription of the MYC gene, hence its stabilization by GD-07 blocks transcription which reduces MYC expression and prevents the metabolic rewiring required for cancer cell survival.
“GD 07 inhibits MYC transcription and translation, demonstrating superior efficacy in ovarian cancer patient-derived organoids. These findings highlight its strong translational potential for managing ovarian cancer and offer a promising strategy for future MYC-targeted therapeutic development.” – Dr. Vinit Kumar
What are the potential implications of your findings for the field and society?
- An alternative approach to target MYC: Targeting the promoter G-quadruplex offers a tractable strategy when direct protein inhibition is difficult.
- A strategy with significant therapeutic potential in ovarian cancer: GD-07 outperformed carboplatin in multiple patient-derived organoid models indicating promising therapeutic potential and supports further preclinical development.
- Built on safer design principles and selectivity: Sequence-informed binding and weak duplex-DNA interaction suggests a path toward safer and more selective DNA-targeted therapeutics.
What was the exciting moment during your research?
Two standout moments:
- Observing NMR evidence that GD-07 engages the upper/5′ tetrad of the c-MYC G-quadruplex (a clear structural “hit”)
- Observing strong activity in patient-derived organoids, where GD-07 outperformed carboplatin in most models tested.
Paper reference: Singh M. et al. Nature-Inspired MYC Inhibitor Disrupts MYC-Driven Glycolysis and Restricts Ovarian Tumor Growth. ChemMedChem (2025) e202500554. https://doi.org/10.1002/cmdc.202500554
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




