Research Summary: This work builds on our earlier identification that many paralogs of 14-3-3 which are well known to bind to phosphorylated peptides and proteins are also capable of binding and hydrolysing ATP. We now show that ATP allosterically controls the function of 14-3-3 protein in two opposing ways. ATP binding modulates 14-3-3 activity by enhancing ATPase activity and reducing its ability to interact with specific peptide partners in vitro. These results point to ATP as a potential regulator of structure and activity 14-3-3 protein.
Author interview

Priyanka Bagdiya is a dedicated PhD scholar in the protein interaction lab, led by Dr. Prasanna Venkatraman, at Cancer research institute, ACTREC. She is also a recipient of the prestigious Shyama Prasad Mukherjee Fellowship (CSIR-SPMF). Her interest lies in understanding the effect of mutations on conformation, structure and function of proteins.
Lab: Dr. Prasanna Venkatraman, CRI- ACTREC (Homi Bhabha National Institute)
Website: https://prasannalab.wixsite.com/mysite/people
What was the core problem you aimed to solve with this research?
Our research was focused on identifying the ATP binding and hydrolysis sites in 14-3-3, a hub signalling protein involved in a wide range of crucial cellular functions. The problem was significant because the 14-3-3 protein is an all alfa helical protein and does not carry any conserved motifs or binding sites known to be involved in such a function. The second problem was to really find out the importance of this enzymatic role in the more canonical functions of 14-3-3 and try to understand the implication of the proposed dual ATP binding sites.
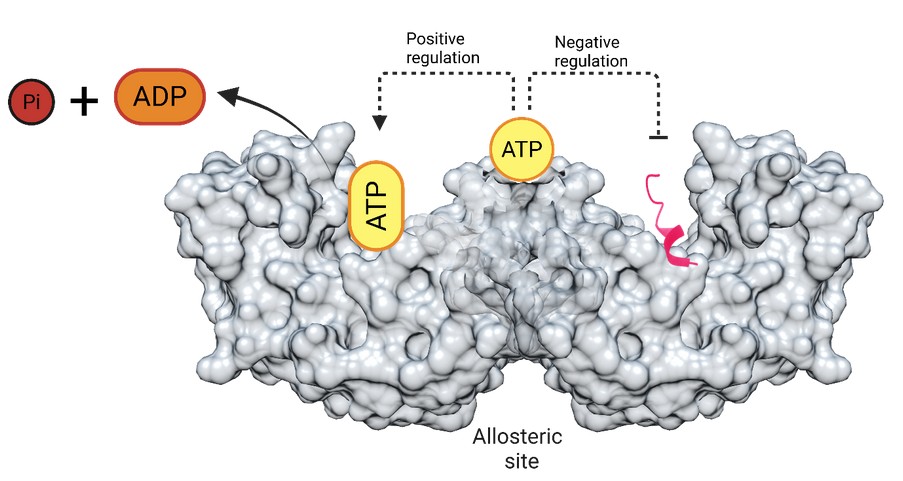
How did you go about solving this problem?
To solve this problem, we used a combination of computational, structural, biochemical and biophysical approaches. We first used computational modelling in collaboration with Dr Madhusudhan’s team in IISER Pune, and LIP-MS (limited proteolysis-mass spectrometry) to predict and identify the ATP binding sites on 14-3-3. These approaches allowed us to map potential ATP-interacting residues and explore how ATP engagement could induce conformational changes. We then experimentally validated these sites using targeted mutagenesis, ATP hydrolysis assays and peptide-binding studies. These combined strategies revealed helped us clearly identify the two ATP sites, assign roles for each of them including an allosteric role for bound ATP at the interface of the protein and how ATP binding negatively regulate 14-3-3’s interaction with peptide partners while promoting its own hydrolysis,
How would you explain your research outcomes (Key findings) to the non-scientific community?
Our research shows that the 14-3-3 protein, which works like a “traffic controller” in the cell, by binding to many different proteins that are modified under specific circumstances. One of the outstanding questions in the field is how such binding events take place. From our results we can speculate that selectivity can be imposed by the energy currency of the cell namely ATP which is known to act as an on and off switch of protein function. When ATP binds to 14-3-3, it changes its shape and we believe that this may inform decisions on whether or not to bind to certain proteins such as the toxins from pathogenic organisms that may hijack 14-3-3 for its own divisive means.
It is one of those studies that raise more questions than answers; the most intriguing of them is the fundamental principles of structure activity relationship and how little we know about the hidden traits of conservation. — Dr. Prasanna Venkatraman
What are the potential implications of your findings for the field and society?
Our findings have several important implications. At the scientific level, the study reveals a previously unrecognized mechanism by which ATP can regulate the function of 14-3-3 protein. 14-3-3 protein is a key signalling hub, which plays an important role in crucial cellular processes like cell cycle, metabolism, apoptosis etc. 14-3-3 protein is crucial for the stabilization of exotoxins secreted by bacteria in human cells. Through allosteric control, ATP specifically inhibits the interaction of such exotoxin peptides and can be used to develop a specific drug against such bacterial infections. It is possible that there are other proteins which carry a similar sequence that undergo regulation by this mechanism. These could then be under metabolic regulation where ATP plays a central role, From a societal and biomedical perspective, since 14-3-3 is involved in processes such as cell survival, stress response, and disease-related signaling, understanding its regulation could eventually help in designing targeted therapeutics for conditions like cancer, neurodegenerative disorders, or metabolic diseases where 14-3-3 function is disrupted.
What was the exciting moment during your research?
One of the most exciting moments came when our computational predictions and LIP-MS experiments perfectly aligned, pinpointing the ATP binding and hydrolysis sites on 14-3-3. It was even more thrilling when we identified a mutant that completely lost ATPase activity, confirming not only the accuracy of our predictions but also the functional importance of these sites. Seeing the experiments validate the models in such a clear way felt like a major breakthrough in the project. Another memorable moment was realizing that ATP binding didn’t just occupy the site, it actively changed the protein’s behavior. It was particularly exciting to identify a peptide from Pseudomonas aeruginosa Exotoxin, whose binding was affected by ATP, highlighting a concrete example of how ATP can modulate 14-3-3 interactions in a biologically meaningful way.
Paper reference: Bagdiya P, Soni N, Jaiswal D, Dalvi S, Kanitkar T, Madhusudhan MS, Venkatraman P. ATP-Driven Allosteric Regulation of 14-3-3: Positive Modulation of ATP Hydrolysis and Negative Regulation of Peptide Binding. FASEB J. 2025 Sep 30;39(18):e70985. https://doi.org/10.1096/fj.202500445R
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
📢 Advertise with BioPatrika – Reach the Right Audience, Fast!
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




