Research Summary: Rotenone alters microtubule dynamics, increasing mitochondrial ROS and nuclear DNA damage. PARP1 hyperactivation triggers HMGB1 nuclear export leading to impairment of DNA damage response and prolonged G2/M cell cycle arrest.
Author interview

Sourav Dutta is a PhD student at Institute of Health Sciences, Presidency University, Kolkata. His research focuses on the regulation of HMGB1 in cell cycle and neuronal cell death under mitochondrial stress response.
Linkedin: www.linkedin.com/in/souravsdbio
Twitter: https://x.com/SouravSDbio
Instagram: Instagram
Lab: Dr. Piyali Mukherjee, Presidency University, Kolkata
What was the core problem you aimed to solve with this research? High mobility group box protein 1 (HMGB1) has a well described role in inflammation when it comes out in the cytoplasm or extracellular milieu from the nucleus in response to inflammatory stimuli like LPS or pathogenic infection. Despite being the second most abundant nuclear protein and most abundant non-histone nuclear protein, its role within the nucleus for maintaining genomic stability is vaguely understood. Extracellular HMGB1 levels in Parkinson’s disease patients serum has been correlated with increased neuro-inflammation and neurodegeneration, but the possible adversities of the nuclear loss of HMGB1 have remained ignored. We tried to understand the regulation of nuclear HMGB1 and its implication in neuronal cell death upon exposure to the environmental toxin rotenone that is known to cause sporadic cases of PD.
How did you go about solving this problem?
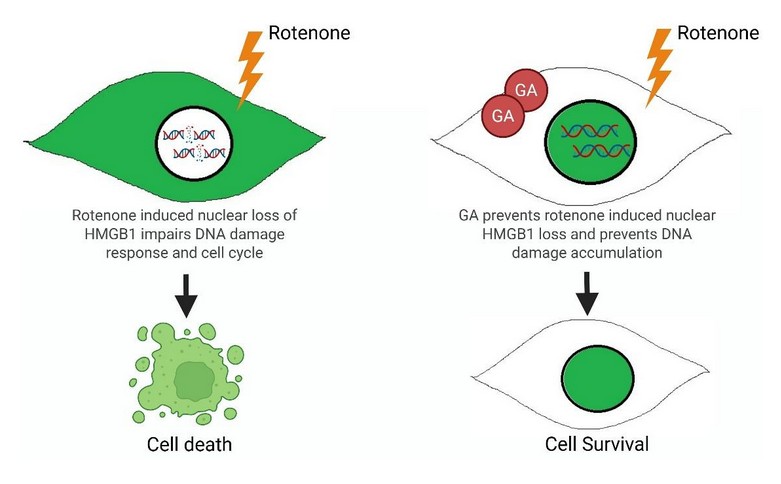
We first determined the subcellular localization of HMGB1 upon rotenone exposure in SH-SY5Y cells, a neuroblastoma cell line and a widely used model for PD studies in vitro. The robustness of HMGB1 nuclear export upon rotenone treatment intrigued us to uncover the underlying mechanism. Naturally we first hypothesized, this HMGB1 release would trigger inflammatory cytokines, but to our surprise it didn’t affect the gene expression of inflammatory cytokines. After a long setback, our focus finally shifted towards the condensed chromatin structures in the rotenone treated cell, indicating mitosis. Revisiting all the previous confocal images made us confident of the correlation of HMGB1 nuclear export and G2/M cell cycle arrest. Further preventing HMGB1 nuclear export by pharmacological inhibition and acetylation of dead mutant prominently blocked rotenone induced G2/M arrest. Confocal imaging and gene knockdown studies showed rotenone increased tubulin hyperacetylation and triggered HMGB1 release. To differentiate the effect of rotenone from other known mitotic blockers we used nocodazole and found, rotenone treated cells fail to resolve DNA damage and re-enter cell cycle even after its removal unlike nocodazole, implicating an irreversible and prolonged G2/M arrest. Further ChIP-Seq re-analysis and gene expression analysis revealed, loss of nuclear HMGB1 potentially impairs DNA damage repair response and prevents nuclear HMGB1 loss ameliorated rotenone induced DNA damage.
How would you explain your research outcomes (Key findings) to the non-scientific community?
Rotenone is a pesticide known to cause Parkinson’s disease by targeting mitochondria, the powerhouse of the cell. Disruption of mitochondria can increase reactive oxygen species (ROS), which damages DNA, the genetic code of the cell, causing mutations. When cells sense any damage in the DNA it tries to stop the cell division and repair the damaged DNA to prevent any mutation to proceed further down the line. Further, if the damage is beyond repair, cells undergo a self-destructive mechanism leading to cell death. There are various factors that help in this process of DNA damage repair. We found HMGB1 may potentially regulate the expression of various other factors involved in the repair mechanism and its depletion from nucleus halts the repair and blocks the cells from dividing further. Rotenone triggers this depletion of nuclear HMGB1 and increases DNA damage to a state beyond repair, leading to death of neuronal cells that are important for memory storage and serve as the messenger cells in the brain for speech, movement, feel and everything we do.
This study uncovers a non-canonical role of HMGB1 in G2/M cell-cycle arrest triggered by mtROS and tubulin hyperacetylation in neuronal cells. — Dr. Piyali Mukherjee
What are the potential implications of your findings for the field and society?
With the increase in aging population age-associated neurodegenerative diseases like Parkinson’s disease (PD) are on the rise. Even after decades of research, there is still no cure for such debilitating conditions. While scientists have focused mostly on protein aggregation like alpha synucleinopathy as the primary drivers of this disease, it is time to take a step back and look at early events, driving disease onset. In this study, using various time-dependent analysis we have dissected the early and late events associated with rotenone-induced neurotoxicity. We encounter DNA damage in our daily lives that could be due to both extrinsic and intrinsic factors like ROS. As accumulation of DNA damage can serve as a deciding factor of cell fate, it is important to dissect the mechanism of DNA damage repair. Here we have uncovered the role of tubulin hyperacetylation-mtROS-HMGB1, a novel axis in the regulation of DNA damage repair and cell cycle progression in neuronal cells. Our study further emphasizes the differential regulation of neurons and non-neuronal cells like glia in the brain, in response to neurodegenerative stresses like rotenone that could take us a step forward in designing targeted therapy for this disease and other neurodegenerative conditions.
What was the exciting moment during your research?
When I first saw the glycyrrhizic acid treated cells under the microscope and discovered that preventing nuclear export of HMGB1 by this inhibitor blocked rotenone induced G2/M cell cycle arrest. It is one the best moments of my PhD journey till now. This observation highlighted the tremendous impact of HMGB1 in rotenone induced neuronal cell death and emphasized the potential it carries as a possible therapeutic target for further exploration in neurodegenerative diseases.
Paper reference: Tubulin hyperacetylation drives HMGB1 nuclear exit via the ROS-PARP1 axis, leading to rotenone-induced G2/M arrest Dutta, Sourav et al. Journal of Biological Chemistry, Volume 301, Issue 10, 110695. https://www.jbc.org/article/S0021-9258(25)02547-5/fulltext
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




