Iron-deplete diet enhances worm lifespan
Research Summary: The study reveals that gut microbes extend Caenorhabditis elegans lifespan by inducing oxidative stress, which disrupts iron homeostasis. An iron-depleted diet produces similar effects, highlighting iron metabolism’s role in longevity.
Author interview

Priyanka Das is a PhD scholar in Dr. Jogender Singh’s lab at IISER Mohali. Her research focuses on identifying microbial metabolites that can enhance host lifespan.
Twitter: https://x.com/_Das_Priyanka
Lab: Dr. Jogender Singh, IISER Mohali
Lab social media: https://x.com/Jogender_Singh7
Website: https://sites.google.com/view/jogendersinghlab/
What was the core problem you aimed to solve with this research?
The gut microbiota of an organism plays a crucial role in maintaining host health and lifespan, and its dysbiosis has been linked to aging and age-related disorders. However, the specific microbial metabolites that can influence lifespan remain poorly understood. Our study aimed to identify novel microbial metabolites that enhance host lifespan and to elucidate their mechanisms of action.
How did you go about solving this problem?
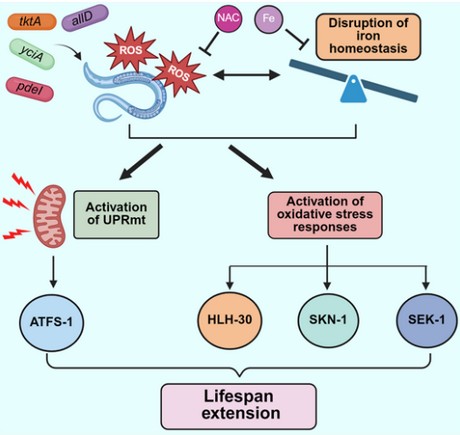
To identify microbial metabolites that enhance lifespan, we used the nematode C. elegans as the host and a genome-wide Escherichia coli mutant library as a surrogate microbiota. The idea was to feed C. elegans different E. coli mutants, identify those that extend lifespan, and then determine which bacterial metabolites drive this effect. However, the library contains about 4,000 mutants, making direct lifespan assays for each one impractical. To overcome this, we employed C. elegans reporter strains as proxies for lifespan changes. Specifically, we used a strain that reports expression of fat-7, a gene involved in converting saturated fatty acids into monounsaturated fatty acids, whose expression levels are known to influence lifespan.
From this screen, we identified 26 E. coli mutants that suppressed fat-7 expression. Interestingly, all of these mutants also significantly extended C. elegans lifespan. We found that these mutants induced oxidative stress in the host, and that this mild oxidative stress, despite generally being considered harmful, had beneficial, lifespan-extending effects. Further analysis revealed that oxidative stress disrupted iron homeostasis, reducing bioavailable iron. Restricting dietary iron similarly increased lifespan. Finally, we demonstrated that activation of host oxidative stress-response pathways in response to the mutant E. coli and iron limitation accounted for the observed lifespan extension.
How would you explain your research outcomes (Key findings) to the non-scientific community?
We wanted to understand whether certain gut bacteria, or the chemicals they produce, could help animals live longer. To test this, we used a tiny worm called C. elegans, a common research organism because its biology is well understood and it ages quickly.
From our screen, we identified 26 mutants of the bacterium E. coli that increased the worms’ lifespan. These bacteria triggered a small amount of oxidative stress inside the worms, a type of stress usually considered harmful. However, in this case, the stress was mild enough to activate the worms’ natural defense systems, making them more resilient and helping them live longer.
This stress also affected how the worms handled iron, reducing the amount of iron available in their bodies. When we lowered the iron content in the worms’ diet, they also lived longer, suggesting that reduced iron levels on their own can promote longevity.
Overall, our results indicate that certain bacteria can activate beneficial stress responses and influence how nutrients like iron are regulated, both of which can contribute to a longer, healthier life.
What are the potential implications of your findings for the field and society?
Gut bacteria are known to influence host physiology, including lifespan. However, the specific mechanisms and metabolites through which gut bacteria affect lifespan remain poorly understood. Our study addresses this gap by characterizing how gut bacteria can increase host lifespan.
We also demonstrate the successful use of reporter strains as proxies for lifespan, enabling high-throughput, high-resolution screens. In the future, different stress-response reporter strains could be used to identify additional bacterial metabolites and mechanisms that promote longevity.
Furthermore, our study highlights that mild oxidative stress can have beneficial effects on the host. This finding challenges the conventional view that oxidative stress is purely harmful, underscoring the need to reconsider the widespread use of antioxidants and encouraging further research into their role in health and lifespan.
Our findings reveal a bacterial-host metabolic axis that links oxidative stress, iron homeostasis, and longevity in C. elegans. — Dr. Jogender Singh
What was the exciting moment during your research?
We were testing the hypothesis that a reporter strain could serve as a proxy for lifespan. The most exciting moment in our research came when we discovered that all E. coli mutants that reduced C. elegans fat-7 expression also extended its lifespan. This finding supported our hypothesis that reporter strains can be used for high-throughput, high-resolution screens to identify bacterial mutants that promote host longevity, ultimately leading to the discovery of 26 novel pro-longevity E. coli mutants.
Another exciting moment occurred when we compared the transcriptomic data of C. elegans fed different bacterial mutants. We observed a remarkable overlap in the genes that were differentially regulated across these diets, suggesting that all the mutants act through the same underlying molecular mechanism.
Paper reference: Das, P., Ravi, & Singh, J. (2025). Iron-deplete diet enhances Caenorhabditis elegans lifespan via oxidative stress response pathways. The EMBO Journal. https://doi.org/10.1038/s44318-025-00634-7
Explore more
🎤 Career – Real career stories and job profiles of life science professionals. Discover current opportunities for students and researchers.
💼 Jobs – The latest job openings and internship alerts across academia and industry.
🛠️ Services – Regulatory support, patent filing assistance, and career consulting services.




